Skin infections are amongst the most common infectious diseases, ranked as the fourth leading cause of human illnesses. Both bacteria and viruses are responsible for many serious, difficult to treat skin conditions. Fungi are also highly prevalent in skin diseases. Skin infections present considerable threats to a person’s health, psychological wellbeing, capacity to operate, and social involvement.
- pathogenic microorganisms
- disruption of skin functions
- biomolecule-based treatments
- mechanisms of action
- prevalent skin infectious diseases
1. Overview
In assigning priorities, skin infectious diseases are frequently classified as minor when compared to infectious diseases of high mortality rates, such as tuberculosis or HIV. However, skin infections are amongst the most common and prevalent diseases worldwide. Elderly individuals present an increased susceptibility to skin infections, which may develop atypical signs and symptoms or even complicate pre-existing chronic disorders. When the skin fails to correct or inhibit the action of certain pathogenic microorganisms, biomolecules endowed with antimicrobial features are frequently administered topically or systemically to assist or treat such conditions. (1) Antibiotics, (2) antimicrobial peptides, or (3) natural extracts display important features that can actively inhibit the propagation of these pathogens and prevent the evolution of infectious diseases. This review highlights the properties and mechanisms of action of these biomolecules, emphasizing their effects on the most prevalent and difficult to treat skin infections caused by pathogenic bacteria, fungi, and viruses. The versatility of biomolecules’ actions, their symbiotic effects with skin cells and other inherent antimicrobial components, and their target-directed signatures are also explored here.
2. Skin Infections
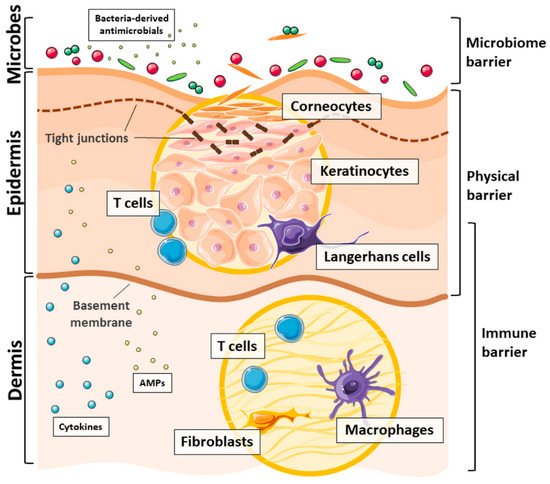
3. Skin: The First Line of Defense
4. Conclusions
The response of the skin to microbial pathogen invasion is a highly coordinated phenomena that encompasses all skin layers and, in many cases, the skin microbiota. At a molecular level, the microbiota-produced antimicrobial factors, the host AMPs, and the antimicrobial lipids play a crucial role in fighting pathogenicity. However, the skin, on its own, is not always effective in fighting the penetration of bacteria, fungi, or viruses, and, as such, serious skin infectious may occur. Antimicrobial biomolecules, including antibiotics, AMPs, or plant extracts, may assist in overcoming this limitation. Their propensity to both control infections, eliminating pathogenic microorganisms, and be involved in important anti-inflammatory actions has rendered these biomolecules essential in medicine. Indeed, while inducing infection-solving immunity and regulating innate immunity, these antimicrobial biomolecules dampen and potentially damage pro-inflammatory reactions. Their varied roles and synergisms with innate substrates have been highlighted in the treatment of many skin infectious diseases. This review explored conventional and more recent therapeutic strategies based on active biomolecules to treat some of the most recurrent skin infections. Even though many do not consider skin infections a global problem and do not demand urgent solutions, modern research continues to dedicate its resources to finding more effective strategies that present the smallest impact on human health or our natural reserves.References
- Rohr, J.R.; Barrett, C.B.; Civitello, D.J.; Craft, M.E.; Delius, B.; DeLeo, G.A.; Hudson, P.J.; Jouanard, N.; Nguyen, K.H.; Ostfeld, R.S. Emerging human infectious diseases and the links to global food production. Nat. Sustain. 2019, 2, 445–456.
- Jones, K.E.; Patel, N.G.; Levy, M.A.; Storeygard, A.; Balk, D.; Gittleman, J.L.; Daszak, P. Global trends in emerging infectious diseases. Nature 2008, 451, 990–993.
- Kumar, S.; Bhatt, M.L.; Saxena, S.K. Global trends in emerging viral infectious diseases: Challenges to the mankind. RASSA J. Sci. Soc. 2019, 1, 7–12.
- Bloom, D.E.; Black, S.; Rappuoli, R. Emerging infectious diseases: A proactive approach. Proc. Natl. Acad. Sci. USA 2017, 114, 4055–4059.
- Tizek, L.; Schielein, M.; Seifert, F.; Biedermann, T.; Böhner, A.; Zink, A. Skin diseases are more common than we think: Screening results of an unreferred population at the Munich Oktoberfest. J. Eur. Acad. Dermatol. Venereol. 2019, 33, 1421–1428.
- Seth, D.; Cheldize, K.; Brown, D.; Freeman, E.E. Global burden of skin disease: Inequities and innovations. Curr. Dermatol. Rep. 2017, 6, 204–210.
- Kwiecien, K.; Zegar, A.; Jung, J.; Brzoza, P.; Kwitniewski, M.; Godlewska, U.; Grygier, B.; Kwiecinska, P.; Morytko, A.; Cichy, J. Architecture of antimicrobial skin defense. Cytokine Growth Factor Rev. 2019, 49, 70–84.
- Tavares, T.D.; Antunes, J.C.; Ferreira, F.; Felgueiras, H.P. Biofunctionalization of natural fiber-reinforced biocomposites for biomedical applications. Biomolecules 2020, 10, 148.
- Kaye, K.S.; Petty, L.A.; Shorr, A.F.; Zilberberg, M.D. Current epidemiology, etiology, and burden of acute skin infections in the United States. Clin. Infect. Dis. 2019, 68, S193–S199.
- Miranda, C.S.; Ribeiro, A.R.; Homem, N.C.; Felgueiras, H.P. Spun Biotextiles in Tissue Engineering and Biomolecules Delivery Systems. Antibiotics 2020, 9, 174.
- Teixeira, M.A.; Amorim, M.T.P.; Felgueiras, H.P. Poly (vinyl alcohol)-based nanofibrous electrospun scaffolds for tissue engineering applications. Polymers 2020, 12, 7.
- Teixeira, M.A.; Paiva, M.C.; Amorim, M.T.P. Electrospun nanocomposites containing cellulose and its derivatives modified with specialized biomolecules for an enhanced wound healing. Nanomater 2020, 10, 557.
- Godlewska, U.; Brzoza, P.; Kwiecień, K.; Kwitniewski, M.; Cichy, J. Metagenomic Studies in Inflammatory Skin Diseases. Curr. Microbiol. 2020, 77, 3201–3212.
- Chen, Y.E.; Fischbach, M.A.; Belkaid, Y. Skin microbiota–host interactions. Nature 2018, 553, 427–436.
- Choi, E.H. Aging of the skin barrier. Clin. Dermatol. 2019, 37, 336–345.
- Lebre, M.C.; van der Aar, A.M.; van Baarsen, L.; van Capel, T.M.; Schuitemaker, J.H.; Kapsenberg, M.L.; de Jong, E.C. Human keratinocytes express functional Toll-like receptor 3, 4, 5, and 9. J. Investig. Dermatol. 2007, 127, 331–341.
- Felgueiras, H.P.; Amorim, M.T.P. Functionalization of electrospun polymeric wound dressings with antimicrobial peptides. Colloids Surf. B 2017, 156, 133–148.
- Fischer, C.L. Antimicrobial activity of host-derived lipids. Antibiotics 2020, 9, 75.
- Strnadova, K.; Sandera, V.; Dvorankova, B.; Kodet, O.; Duskova, M.; Smetana, K.; Lacina, L. Skin aging: The dermal perspective. Clin. Dermatol 2019, 37, 326–335.
- Felgueiras, H.P.; Teixeira, M.A.; Tavares, T.D.; Homem, N.C.; Zille, A.; Amorim, M.T.P. Antimicrobial action and clotting time of thin, hydrated poly (vinyl alcohol)/cellulose acetate films functionalized with LL37 for prospective wound-healing applications. J. Appl. Polym. Sci. 2020, 137, 48626.
- Graham, H.K.; Eckersley, A.; Ozols, M.; Mellody, K.T.; Sherratt, M.J. Human Skin: Composition, Structure and Visualisation Methods. In Skin Biophysics; Springer: Berlin/Heidelberg, Germany, 2019; pp. 1–18.
- Zhang, L.-j.; Guerrero-Juarez, C.F.; Hata, T.; Bapat, S.P.; Ramos, R.; Plikus, M.V.; Gallo, R.L. Dermal adipocytes protect against invasive Staphylococcus aureus skin infection. Science 2015, 347, 67–71.
