Soybeans are rich in proteins and lipids and have become a staple part of the human diet. Besides their nutritional excellence, they have also been shown to contain various functional components, including isoflavones, and have consequently received increasing attention as a functional food item. Isoflavones are structurally similar to 17-β-estradiol and bind to estrogen receptors (ERα and ERβ). The estrogenic activity of isoflavones ranges from a hundredth to a thousandth of that of estrogen itself. Isoflavones play a role in regulating the effects of estrogen in the human body, depending on the situation. Thus, when estrogen is insufficient, isoflavones perform the functions of estrogen, and when estrogen is excessive, isoflavones block the estrogen receptors to which estrogen binds, thus acting as an estrogen antagonist. In particular, estrogen antagonistic activity is important in the breast, endometrium, and prostate, and such antagonistic activity suppresses cancer occurrence.
- soybean-derived isoflavones
- physiological effect
- gene regulation
- health benefit mechanism
1. Introduction
2. Content, Structure, and Metabolism of Isoflavones
2.1. Isoflavone Content in Soybean
2.2. Isoflavone Biosynthesis and Regulation
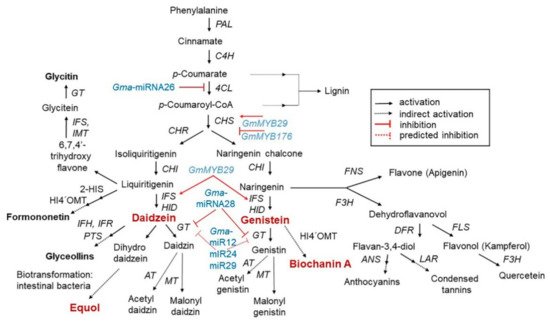
2.3. Structure and Type of Isoflavones
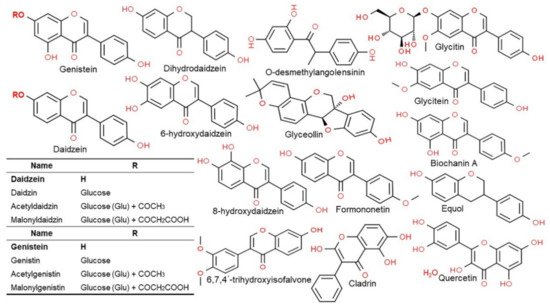
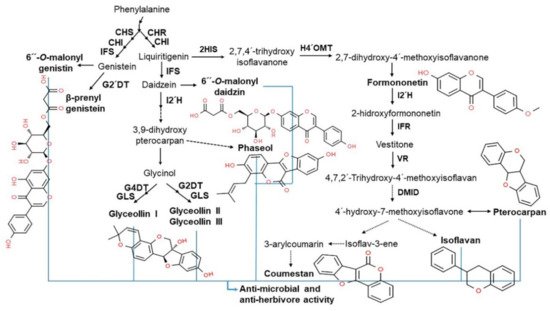
2.4. Functionality of Isoflavones in Plants
References
- Calaf, G.M.; Ponce-Cusi, R.; Aguayo, F.; Munoz, J.P.; Bleak, T.C. Endocrine disruptors from the environment affecting breast cancer. Oncol. Lett. 2020, 20, 19–32.
- Sirtori, C.R. Risks and benefits of soy phytoestrogens in cardiovascular diseases, cancer, climacteric symptoms and osteoporosis. Drug. Saf. 2001, 24, 665–682.
- Petrine, J.C.P.; Del Bianco-Borges, B. The influence of phytoestrogens on different physiological and pathological processes: An overview. Phytother. Res. 2021, 35, 180–197.
- Bacciottini, L.; Falchetti, A.; Pampaloni, B.; Bartolini, E.; Carossino, A.M.; Brandi, M.L. Phytoestrogens: Food or drug? Clin. Cases Miner. Bone Metab. 2007, 4, 123–130.
- Krizova, L.; Dadakova, K.; Kasparovska, J.; Kasparovsky, T. Isoflavones. Molecules 2019, 24, 1076.
- Pabich, M.; Materska, M. Biological effect of soy isoflavones in the prevention of civilization diseases. Nutrients 2019, 11, 1660.
- Kim, I.S.; Hwang, C.W.; Yang, W.S.; Kim, C.H. Current perspectives on the physiological activities of fermented soybean-derived cheonggukjang. Int. J. Mol. Sci. 2021, 22, 5746.
- Kim, I.S.; Kim, C.H.; Yang, W.S. Physiologically active molecules and functional properties of soybeans in human health—A current perspective. Int. J. Mol. Sci. 2021, 22, 4054.
- Szeja, W.; Grynkiewicz, G.; Rusin, A. Isoflavones, their glycosides and glycoconjugates. Synthesis and biological activity. Curr. Org. Chem. 2017, 21, 218–235.
- Kim, J.S.; Lee, J.H.; Surh, J.; Kang, S.A.; Jang, K.H. Aglycone isoflavones and exopolysaccharides produced by Lactobacillus acidophilus in fermented soybean paste. Prev. Nutr. Food Sci. 2016, 21, 117–123.
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47.
- Chen, L.R.; Ko, N.Y.; Chen, K.H. Isoflavone supplements for menopausal women: A Systematic review. Nutrients 2019, 11, 2649.
- Miadokova, E. Isoflavonoids—An overview of their biological activities and potential health benefits. Interdiscip. Toxicol. 2009, 2, 211–218.
- Lecomte, S.; Demay, F.; Ferriere, F.; Pakdel, F. Phytochemicals targeting estrogen receptors: Beneficial rather than adverse effects? Int. J. Mol. Sci. 2017, 18, 1381.
- Vitale, D.C.; Piazza, C.; Melilli, B.; Drago, F.; Salomone, S. Isoflavones: Estrogenic activity, biological effect and bioavailability. Eur. J. Drug Metab. Pharmacokinet. 2013, 38, 15–25.
- He, F.J.; Chen, J.Q. Consumption of soybean, soy foods, soy isoflavones and breast cancer incidence: Differences between Chinese women and women in Western countries and possible mechanisms. Food Sci. Hum. Well. 2013, 2, 146–161.
- Garcia-Calderon, M.; Perez-Delgado, C.M.; Palove-Balang, P.; Betti, M.; Marquez, A.J. Flavonoids and isoflavonoids biosynthesis in the model legume Lotus japonicus; Connections to nitrogen metabolism and photorespiration. Plants 2020, 9, 774.
- Gupta, O.P.; Nigam, D.; Dahuja, A.; Kumar, S.; Vinutha, T.; Sachdev, A.; Praveen, S. Regulation of isoflavone biosynthesis by miRNAs in two contrasting soybean genotypes at different seed developmental stages. Front. Plant Sci. 2017, 8, 567.
- Yu, O.; Jung, W.; Shi, J.; Croes, R.A.; Fader, G.M.; McGonigle, B.; Odell, J.T. Production of the isoflavones genistein and daidzein in non-legume dicot and monocot tissues. Plant Physiol. 2000, 124, 781–794.
- Akashi, T.; Aoki, T.; Ayabe, S. Molecular and biochemical characterization of 2-hydroxyisoflavanone dehydratase. Involvement of carboxylesterase-like proteins in leguminous isoflavone biosynthesis. Plant Physiol. 2005, 137, 882–891.
- Ahmad, M.Z.; Li, P.; Wang, J.; Rehman, N.U.; Zhao, J. Isoflavone malonyltransferases GmIMaT1 and GmIMaT3 differently modify isoflavone glucosides in soybean (Glycine max) under various stresses. Front. Plant Sci. 2017, 8, 735.
- Sugiyama, A.; Yamazaki, Y.; Hamamoto, S.; Takase, H.; Yazaki, K. Synthesis and secretion of isoflavones by field-grown soybean. Plant Cell Physiol. 2017, 58, 1594–1600.
- Falcone Ferreyra, M.L.; Rius, S.P.; Casati, P. Flavonoids: Biosynthesis, biological functions, and biotechnological applications. Front. Plant Sci. 2012, 3, 222.
- Dong, N.Q.; Lin, H.X. Contribution of phenylpropanoid metabolism to plant development and plant-environment interactions. J. Integr. Plant Biol. 2021, 63, 180–209.
- Sarkar, M.A.R.; Watanabe, S.; Suzuki, A.; Hashimoto, F.; Anai, T. Identification of novel MYB transcription factors involved in the isoflavone biosynthetic pathway by using the combination screening system with agroinfiltration and hairy root transformation. Plant Biotechnol. 2019, 36, 241–251.
- Chu, S.; Wang, J.; Zhu, Y.; Liu, S.; Zhou, X.; Zhang, H.; Wang, C.E.; Yang, W.; Tian, Z.; Cheng, H.; et al. An R2R3-type MYB transcription factor, GmMYB29, regulates isoflavone biosynthesis in soybean. PLoS Genet. 2017, 13, e1006770.
- Izumi, T.; Piskula, M.K.; Osawa, S.; Obata, A.; Tobe, K.; Saito, M.; Kataoka, S.; Kubota, Y.; Kikuchi, M. Soy isoflavone aglycones are absorbed faster and in higher amounts than their glucosides in humans. J. Nutr. 2000, 130, 1695–1699.
- Andres, S.; Hansen, U.; Niemann, B.; Palavinskas, R.; Lampen, A. Determination of the isoflavone composition and estrogenic activity of commercial dietary supplements based on soy or red clover. Food Funct. 2015, 6, 2017–2025.
- Dixon, R.A. Natural products and plant disease resistance. Nature 2001, 411, 843–847.
- Singh, S.; Kaur, I.; Kariyat, R. The Multifunctional roles of polyphenols in plant-herbivore interactions. Int. J. Mol. Sci. 2021, 22, 1442.
- Jayaraman, D.; Forshey, K.L.; Grimsrud, P.A.; Ane, J.M. Leveraging proteomics to understand plant-microbe interactions. Front. Plant Sci. 2012, 3, 44.
- Olanrewaju, O.S.; Ayangbenro, A.S.; Glick, B.R.; Babalola, O.O. Plant health: Feedback effect of root exudates-rhizobiome interactions. Appl. Microbiol. Biotechnol. 2019, 103, 1155–1166.
- Sukumaran, A.; McDowell, T.; Chen, L.; Renaud, J.; Dhaubhadel, S. Isoflavonoid-specific prenyltransferase gene family in soybean: GmPT01, a pterocarpan 2-dimethylallyltransferase involved in glyceollin biosynthesis. Plant J. 2018, 96, 966–981.
- Yu, J.; Bi, X.; Yu, B.; Chen, D. Isoflavones: Anti-inflammatory benefit and possible caveats. Nutrients 2016, 8, 361.
- Testa, I.; Salvatori, C.; Di Cara, G.; Latini, A.; Frati, F.; Troiani, S.; Principi, N.; Esposito, S. Soy-based infant formula: Are phyto-oestrogens still in doubt? Front. Nutr. 2018, 5, 110.
- Dutta, S.; Mitra, M.; Agarwal, P.; Mahapatra, K.; De, S.; Sett, U.; Roy, S. Oxidative and genotoxic damages in plants in response to heavy metal stress and maintenance of genome stability. Plant Signal. Behav. 2018, 13, e1460048.
- Skalicky, M.; Kubes, J.; Hejnak, V.; Tumova, L.; Martinkova, J.; Martin, J.; Hnilickova, H. Isoflavones production and possible mechanism of their exudation in Genista tinctoria L. suspension culture after treatment with vanadium compounds. Molecules 2018, 23, 1619.
- Kubes, J.; Skalicky, M.; Tumova, L.; Martin, J.; Hejnak, V.; Martinkova, J. Vanadium elicitation of Trifolium pratense L. cell culture and possible pathways of produced isoflavones transport across the plasma membrane. Plant Cell Rep. 2019, 38, 657–671.
- Pessoa, J.C.; Etcheverry, S.; Gambino, D. Vanadium compounds in medicine. Coord. Chem. Rev. 2015, 301, 24–48.
- Romera, F.J.; Garcia, M.J.; Lucena, C.; Martinez-Medina, A.; Aparicio, M.A.; Ramos, J.; Alcantara, E.; Angulo, M.; Perez-Vicente, R. Induced systemic resistance (ISR) and Fe deficiency responses in dicot plants. Front. Plant Sci. 2019, 10, 287.
- Conrath, U. Systemic acquired resistance. Plant Signal. Behav. 2006, 1, 179–184.
- Jaiswal, S.K.; Mohammed, M.; Ibny, F.Y.I.; Dakora, F.D. Rhizobia as a source of plant growth-promoting molecules: Potential applications and possible operational mechanisms. Front. Sustain. Food Syst. 2021, 4, 619676.
- Toth, K.; Stacey, G. Does plant immunity play a critical role during initiation of the legume-rhizobium symbiosis? Front. Plant Sci. 2015, 6, 401.
- Cowan, M.M. Plant products as antimicrobial agents. Clin. Microbiol. Rev. 1999, 12, 564–582.
- Gonzalez-Lamothe, R.; Mitchell, G.; Gattuso, M.; Diarra, M.S.; Malouin, F.; Bouarab, K. Plant antimicrobial agents and their effects on plant and human pathogens. Int. J. Mol. Sci. 2009, 10, 3400–3419.
- Lygin, A.V.; Zernova, O.V.; Hill, C.B.; Kholina, N.A.; Widholm, J.M.; Hartman, G.L.; Lozovaya, V.V. Glyceollin is an important component of soybean plant defense against Phytophthora sojae and Macrophomina phaseolina. Phytopathology 2013, 103, 984–994.
- Abdel-Lateif, K.; Bogusz, D.; Hocher, V. The role of flavonoids in the establishment of plant roots endosymbioses with arbuscular mycorrhiza fungi, rhizobia and Frankia bacteria. Plant Signal. Behav. 2012, 7, 636–641.
- Sugiyama, A. The soybean rhizosphere: Metabolites, microbes, and beyond—A review. J. Adv. Res. 2019, 19, 67–73.
