Hemp (Cannabis sativa L.) is an ancient, widespread, multipurpose crop cultivated all over the world, all parts of which can be potentially usable for the production of many different commodities with industrial interest. It is one of the oldest cultivated crops and until the first half of the 1900s, it was widely grown essentially as a fibre crop. Declining the demand for natural fibre consequential to the upper hand of synthetic fibre, and competition from other plant fibre sources led to reduce the demand for hemp. In addition, the use of some narcotic strains containing high and unhealthy level (>0.3%) of the only one psychoactive substance, namely the cannabinoid delta-9 tetrahydrocannabinol (THC), led to the crop's prohibition during much of the 20th century. The clarification of the genetics and the biosynthetic pathway of the cannabinoids has been essential to identify three main different genotypes and the related chemical phenotypes of hemp plants, differing in the content of the two main hemp cannabinoids, THC and cannabidiol (CBD). Among these, the chemical phenotype commonly named "industrial hemp" includes hemp varieties which contain <0.3% or 0.2% of THC level that makes them unsuitable for narcotic purposes, but very useful for many other industrial applications. Therefore, from a legislative point of view, the main western countries such as United States, Canada, and European Union after a prohibition period, from the last decade of 1900s, have reintroduced and restored the industrial hemp cultivation.
- Cannabis sativa L.
- Industrial hemp
- Chemical phenotypes
- Cannabinoids
- THC
- Cannabis sativa L. legislation
1. Definition
Cannabis sativa L., commonly known as hemp, is an herbaceous, anemophilous plant belonging to the Cannabaceae family.
2. History
It is considered one of the most ancient cultivated plants and due to its long history of cultivation, it is difficult to identify its exact centre of origin. According to phylogenetic studies based on molecular analysis and studies on sequence homology of ancient and modern DNA extracted from archaeobotanical and modern samples, respectively, most researchers agreed that this plant species originated in central Asia and was introduced in Europe as a cultivated and domesticated agricultural plant during the Bronze age (approximately, from the 22th until 16th century BC) [1,2][1][2]. Nevertheless, a recent work by McPartland and colleagues[2] [2] provided evidence that C. sativa was indigenous also to Europe. Currently, there are no more traces of wild-type hemp and only domesticated (i.e., individuals of a species chosen and selected by humans for characteristics making them useful to people) and ruderal (i.e., forms growing outside of cultivation) hemp plants exist. Independently to its origin, the nowadays-domesticated form of C. sativa L. is widespread and cultivated not only in the Asian countries, but also in Canada, the United States (US), Europe, and Africa. It is a multipurpose, sustainable, and low environmental impact crop which can be useful for several application fields, from the agricultural and phytoremediation to food and feed, cosmetic, building, and pharmaceutical industries. Indeed, from this highly versatile plant, it is possible to obtain various products of industrial interest such as fibre and shives; bio-building and thermal insulated materials; seeds, flour and oil with important nutritional and functional features; and bioactive compounds of pharmacological interest[3] [3](Figure (Figure 11).
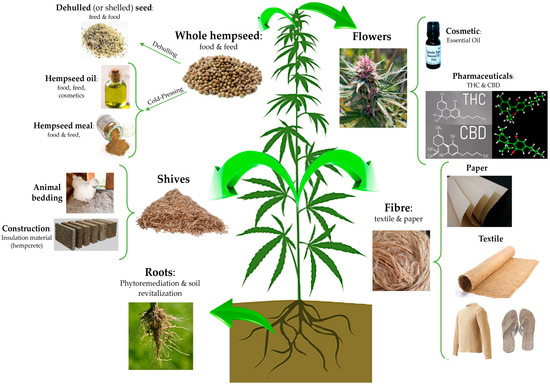
Figure 1. The manifold applications of hemp plant: virtually, each part of this plant can be used in a specific industrial field. The seeds can be used in the food, feed, and cosmetical field as whole or dehulled, or it may be subjected to a cold press process to obtain an oil used in the food and cosmetic industries. From the stem, it is possible to obtain both shives and fibre, useful for animal, building, paper and textile applications. The hemp root system is highly developed in comparison to other herbaceous plants, and this feature is suitable for the phytoremediation of soil from heavy metals. Hemp flowers can be used for ornamental purposes or to obtain products of cosmetic and pharmaceutical interest, such as essential oils composed by delta-9-tetrahydrocannabinol (THC) and cannabidiol (CBD) pure extracts.
Essentially, C. sativa L. can be grown for three main purposes: industrial, narcotic/recreational, and medicinal[4] [4]. Traditionally, C. sativa L. plants were cultivated primarily as a fibre crop for the production of textiles and ropes, especially in the western world. Despite their high nutritional value, the seeds of this plant were initially considered as a by-product of the fibre production, and hence, they were mainly used as animal feed. From the first half of the 21st century, the cultivation of this crop declined because of the progressive diffusion of synthetic fibres and the use of some narcotic strains of the C. sativa L. plant for the production of intoxicant drugs. Only since the last two decades, there has been a reintroduction of the the C. sativa L. cultivation exclusively for industrial purposes, and in this context, Canada has been the first western country to restore this crop, followed by Europe and the US. Nowadays, a growing interest for the seeds of the C. sativa L. plant, commonly named hempseeds, has been developed due to the increased knowledge about their high nutritional value and potential functionality.
3. The Cannabinoids Synthesis in C. sativa L.
The major discriminant factor related to the different intended uses of C. sativa L. is the level of the two major and more known phytochemicals characteristic of this crop, namely the only one psychoactive and toxicant compound of the plant, THC, and the non-psychoactive cannabidiol (CBD). Both of them belong to the cannabinoids’ class which includes over of 100 secondary metabolites belonging to the family of terpenophenolic compounds, typical of all C. sativa L. plants. These compounds are synthesized, collected, and stored in stalked glandular trichomes, that are specialized tiny secretory epidermal glands [5[5][6],6], which are essentially present and abundant on the inflorescence of the female plant, whilst are present in lower numbers on leaves and stems, and are absent on roots and seeds, therefore, these latter organs do not contain cannabinoids [4,7,8][4][7][8]. A possible presence of cannabinoids in hempseeds could occur during the harvesting process, as a result of physical contact with the resin secreted by the glandular trichomes located on the bracts that surround the seed [9,10][9][10]. Hence, the presence of cannabinoids in hempseed actually represents a contamination, and the level of this contamination depends on both the cultivar (cv) and the cleaning process of the seed. Reasonably, THC contamination in seeds from C. sativa L. varieties which produce a low-THC level—as the industrial hemp varieties—should be extremely low [9][9]; anyway, the adoption of a method for the quantification of the possible cannabinoid’s contamination and the level in hempseed products and food may be appropriate [11,12,13][11][12][13].
C. sativa L. plants grown for an industrial purpose, are cultivated to obtain fibre, seeds, and their derivatives. These plants are popularly called “industrial hemp” or “fibre-type” hemp, and they contain low-THC level (i.e., <0.3 or 0.2%), whereas, C. sativa L. plants cultivated for narcotic/recreational purposes are characterized by high-THC level and those cultivated for medicinal purposes are characterized by high-THC and high-CBD levels.
Several works clarified well the cannabinoids’ biosynthetic pathway [14,15,16,17,18,19][14][15][16][17][18][19]. According to these studies, a common precursor of all the main cannabinoids exists, and it is the cannabigerolic acid (CBGA). In the cytosol, CBGA is converted into the acidic form of the three main cannabinoids, from which other related cannabinoid compounds will originate, namely tetrahydrocannabinol acid (THCA), that in the acidic form has no psychoactive activity; cannabidiolic acid (CBDA); and cannabichromenic acid (CBCA). This conversion is catalysed by an oxidocyclase specific for each cannabinoid (THCA-synthase, CBDA-synthase, and CBCA-synthase, respectively) (Figure 2). Finally, the acidic form of each cannabinoid undergoes non-enzymatic decarboxylation to their neutral and active form, i.e., THC with psychoactive activity, CBD, and CBC that is found at high levels in juvenile plants [20[20][21],21], respectively.
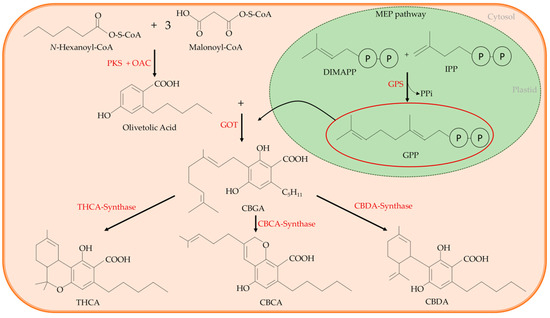
Figure 2. The cannabinoid synthetic pathway: cannabigerolic acid (CBGA) is the common precursor of all main cannabinoids. It is synthesized through an alkylation of the phenolic moiety of olivetolic acid with the terpenoid component of geranyl pyrophosphate (GPP). The reaction is catalysed by a geranylpyrophosphate:olivetolate geranyltransferase (GOT). Olivetolic acid is originated in the cytosolic polyketide pathway through an aldol condensation of hexanoyl-Coenzyme A (CoA) with three molecules of malonyl-CoA, that is catalysed by the polyketide synthase (PKS) enzyme in the presence of olivetolic acid cyclase (OAC). The GPP is synthesized by the plastidial methylerythritol phosphate (MEP) pathway. In the cytosol, CBGA is converted into the acidic form of the three main cannabinoids, tetrahydrocannabinol acid (THCA) that in the acidic form has no psychoactive activity, cannabidiolic acid (CBDA) and cannabichromenic acid (CBCA). GPS: geranyl pyrophosphate synthase; IPP: isopentenyl diphosphate; OAC: olivetolic acid cyclase.
4. Chemical Phenotype and Taxonomy of C. sativa L.
Elucidation of the cannabinoids’ biosynthetic pathway has been essential to demonstrate that the concentration of each cannabinoids in the plant is genetically determined, so that various genotypes related to different chemical phenotypes, diverging in types and concentration of cannabinoids (i.e., cannabinoids profile), exist. These phenotypes are known as “chemotypes” or “biotypes”, and three different principal chemotypes were commonly identified [8,20,22][8][20][22] on the basis of the two main cannabinoids (i.e., THC and CBD) content and ratio:
-
Chemotype I is characterized by a low CBD/THC ratio (0.00–0.05), due to high THC content (>0.3% of dry weight of the reproductive part of the female plant at flowering). This chemical phenotype is also known as “drug type”, “THC-predominant”, or C. sativa L. subsp. Indica and the varieties belonging to this chemotype are those commonly grown for narcotic/recreational purposes.
-
Chemotype II has both the two main cannabinoids, CBD and THC, in a content ratio (CBD/THC) close to the unity (0.5–3.0), usually with a slight prevalence of CBD. This chemical phenotype is also named “intermediate type” or “THC-intermediate”, and the varieties belonging to this chemotype are mainly grown for medicinal use.
-
Chemotype III is characterized by high CBD/THC ratio (15–25) due to high CBD amount and low THC content, not over than 0.3% of dry weight of the reproductive part of the female plant at flowering. This chemical phenotype is also known as “non-drug type”, “fibre-type”, “THC-predominant”, or C. sativa L. subsp. Sativa, and the varieties belonging to this chemotype are cultivated for industrial purposes, namely, for fibre, seeds, and their derivatives.
De Meijer and colleagues[23] [23] for the first time gave a clear genetic meaning to the tripartite distribution of the chemotypes within the C. sativa L. population. Indeed, studying the inheritance of the chemotype traits of C. sativa L. plants, they identified the existence of a single locus named B locus, with two co-dominant alleles, named BT and BD, each of which codes for the THCA- and CBDA-synthases, respectively. Hence, according to this model, the THC-predominant phenotype (chemotype I) is related to the BT/BT genotype, the CBD-predominant phenotype (chemotype III) is determined by the BD/BD genotype, and the intermediate phenotype (chemotype II) is induced by the heterozygous state BT/BD. The same authors also showed that the value of the CBD/THC ratio in the heterozygous hybrids obtained from cross between parental homozygous pure-THC and pure-CBD, unexpectedly differed significantly and deviated from the expected 1.0 value. Therefore, they speculated that some heritable factor could affect the balance between THCA- and CBDA-synthase in their competition to convert the CBGA precursor. In particular, the authors hypothesized that the BT and BD alleles could be part of a wider allelic series coding for several isoenzymatic forms of THCA- and CBDA-synthase, respectively, with differential affinities for the CBGA substrate, resulting in significantly different CBD/THC ratios observed in the heterozygotes. Further studies pointed out the existence of several allelic variants of BD[7][20][24] [7,20,24] and BT[7][8][24][25] [7,8,24,25] genes coding for less or totally non-functional CBDA- and THCA-synthase, respectively, and which therefore, influence the cannabinoid profile of the plant and, from a practical point of view, also represent an useful genetic marker to differentiate the drug-type from the fibre-type C. sativa L. plants. Interestingly, a higher number of allelic variants of wild-type BD locus was found in comparison to the BT locus. Hence, considering the high number of the cannabinoid synthase genes’ allelic variants and the higher mutation rate of the CBDA-synthase allele, Onofri and colleagues[7] [7] proposed a phylogenetic hypothesis according to which it is possible to consider all cannabinoids’ allelic variants as a gene family and to speculate that the wild-type CBDA-synthase allele may be the ancestral form of this gene family, from which events of duplication would have led to a higher CBDA-synthase variation, resulting in the formation of the CBDA-synthase pseudogenes (i.e., the CBDA-synthase allelic variants) and to the rise of a new sequence coding for a new enzyme able to convert the CBDA substrate in a new product, the THCA. According to this evolutionary theory, it has also been hypothesized that the low-functionally THCA-synthase allele found in some fibre type (CBD-predominant) plants [25][25], could be an evolutionarily intermediate between the CBDA-synthase ancestor and the fully functional THCA-synthase allele.
The high intrinsic genetic variability rate of C. sativa L. has been further accentuated by the long history of its domestication. Indeed, the different intended uses of the C. sativa L. cultivation’s products have led over the years, to an artificial phenotypic selection of specific features of the domesticated plants, useful for increasing the yield and/or the quality of the commercial interest’s cultivation products [26][26]. The direct consequence of this selection was the unaware artificial creation of the C. sativa L. varieties, each with specific genotypic and phenotypic features, which at first, induced the taxonomists and botanists to erroneously recognize two or three different species of C. sativa L., embracing a polytypic concept of the Cannabis genus [27][27]. To further complicate the taxonomic classification of the Cannabis genus, there has been also the fact that C. sativa L. is a crop which tends to exist in “crop-weed complexes”, that is complexes of domesticated forms in cultivation and related ruderal (weedy) forms growing outside of cultivation, developing morphological characteristics also very different from those of the domestic progenitor, as a consequence of adaptation to the wild environment [28][28]. However, it must be considered that, despite the high genetic variability of C. sativa L., the varieties that genotypically and phenotypically differ, are interfertile. Therefore, taking into account the Darwinian definition of biological species, “a group of organisms that can reproduce with one another in nature and produce fertile offspring”, C. sativa L. varieties cannot be consider as different species of the Cannabis genus. For this reason, to date, the polytypic concept has been definitely given up and replaced by the monotypic one. According to this, a single species of Cannabis genus exists, namely C. sativa L., which includes several varieties or cultivars (cvs) that genotypically and phenotypically differ, but they all are interfertile and therefore, they belong to the same species [29,30][29][30].
From a practical point of view, the main discrimination factor among the C. sativa L. cvs is the THC content, which, essentially for legal reasons, as described in the next paragraph, is an useful tool to discriminate between the drug-type plants with high THC content, used for medical or recreational purposes, and the fibre-type plants with low THC content, commonly named industrial hemp.
5. Legislation of C. sativa L.
Historically, industrial hemp or simply, hemp, that is, C. sativa L. plants grown for fibre and/or seeds, was frequently cultivated over the world, mainly for the production of technical textiles, until the first half of the 21st century. In the US, hemp was widely grown from the colonial period into the mid-1800s. In the early 1900s and prior to the late 1950s, hemp continued to be grown, being considered as an agricultural commodity: the US Department of Agriculture (USDA) supported its production, and USDA researchers continued to publish information related to hemp production and also reported on hemp’s potential for use in textiles and in paper manufacturing [31][31]. In Europe, at the end of the 1950s, Italy was the second country in the world after Russia for the areas under hemp cultivation (over 100,000 hectares) and was the world’s best for the quality of the obtained products [32][32]. However, following the discovery of the psychotropic activity of THC, and the increasing awareness of its deleterious effects on human health, many countries began to take measures in an effort to stem the use of C. sativa L. plants’ flowers and leaves for their psychotropic effects. The first provision was taken in the US and Canada. In the US, between 1914 and 1933, 33 states passed laws restricting legal production to medicinal and industrial purposes only. In 1937, the Marihuana Tax Act defined hemp as a narcotic drug, without any distinguishing between low THC plants (hemp) and high THC (drug hemp or simply, marijuana) ones: both were considered schedule I controlled substances, and it was required that farmers growing hemp hold a federal registration and special tax stamp. This effectively limited further production expansion; in fact, after 1943, production of hemp started to decline until the late 1950s when no production was recorded. Finally, in 1970, The Controlled Substances Act (CSA) was issued, and it placed the control of selected plants, drugs, and chemical substances under federal jurisdiction. Among the selected plants, there were also C. sativa L. ones to which were given the statutory definition of marijuana and were put in the Drug Enforcement Administration (DEA) schedule of controlled substances [31][31]. In Canada, the cultivation of hemp has been prohibited due to the presence of THC, in 1938 with the Canadian Opium and Narcotics Act [33,34][33][34]. In 1961, the United Nation (UN) endorsed and adopted the single convention on narcotic drugs, which established a universal system for limiting the cultivation, production, distribution, trade, possession, and use of narcotic substances to medical and scientific purposes, with a special focus on plant-derived substances, among which is cannabis. In the article 28, paragraph 2 of this convention, cannabis was defined as “the flowering or fruiting tops of the C. sativa L. plant (excluding the seeds and leaves when not accompanied by the tops) from which the resin has not been extracted, by whatever name they may be designated”. The same article described a system of control required if a country decides to permit the cultivation of C. sativa L. that is not for industrial or horticultural purposes [4,35][4][35]. Ten years later, in 1971, the UN endorsed the convention on psychotropic substances which established an international control system for psychotropic substances, among which is THC [36][36]. In line with these directives, in 1975 the Italian Republic issued the law n. 685/1975, introducing cannabis (intended as a drug product obtained from C. sativa L. plants) in the schedule of controlled substances.
The better knowledge of the biochemical and biomolecular features of the C. sativa L. species has made it possible to understand the genetics and biochemical mechanisms previously described, which are the basis of the cannabinoids’ synthesis and, in particular, of THC. Furthermore, thanks to the development of specific analysis techniques (e.g., gas chromatography or gas chromatography-mass spectrometry), it is now possible to resolutely and accurately quantify the THC content of C. sativa L. plants in order to distinguish between cvs with high and low THC contents. For these reasons, nowadays, the cultivation of industrial hemp has been reintroduced either in the US, Canada, and Europe. Canada was one of the first country to restore industrial hemp cultivation. Indeed, in 1994, it began to issue licenses for hemp as a research crop and then, in 1998, the cultivation of hemp varieties containing less than 0.3% THC of the dry weight of leaves and flowering parts was legalized, and it is currently permitted, provided that a license from the Office of Controlled Substances of Health Canada has been acquired. To date, Canada is the major hemp-producing and -exporting country, particularly of hemp-based foods, ingredients, and other related products [31][31]. In the EU, the hemp cultivation reintroduction took place in 2013 with the EU regulation n. 1307/2013 that allowed the growth of C. sativa L. plants for industrial purposes only for those plants with low levels of THC. According to this regulation, the granting of payments under the Common Agricultural Policy (CAP) is conditional upon the use of certified seeds of specific hemp varieties, that is, C. sativa L. cvs with a THC content not exceeding 0.2% of the dry weight of leaves and flowering parts [4][4]. Thus, the EU has adopted more stringent parameters compared to Canada, to ensure the safety of and to protect the health of citizens. Different genotypes of industrial hemp with a THC content < 0.2% have been selected and registered, and currently, there are about 70 allowed industrial hemp varieties listed in the European Plant Variety Database as agricultural species (Table 1) [37,38][37][38]. Some of these cvs are dioecious as the C. sativa L. plant naturally occurs; other cvs are monoecious and are obtained by ancestral breeding [39][39]. Often, but not always, the monoecious varieties are adopted for seed production since they give a higher yield of the product of interest, whereas the dioecious cvs are mainly adopted for fibre production. Moreover, the industrial hemp varieties listed in the EU plant variety database are constantly updated based on the results of the annual THC-content’s monitoring and on the possible request for the introduction of new cvs with a low THC amount.
Table 1. Industrial hemp cultivars registered and listed in the European Union Plant Variety Database and their origin. All these varieties are certified to contain less than 0.2% of THC of dry weight of leaves and flowering parts of the plant, according to the EU regulation 1307/2013.
| No | Registered Varieties | Origin | No | Registered Varieties | Origin |
|---|---|---|---|---|---|
| 1 | Adzelvieši | Latvia | 33 | Ivory | Nederland |
| 2 | Armanca | Romania | 34 | KC Bonusz | Hungary |
| 3 | Asso | Italy | 35 | KC Dora | Hungary |
| 4 | Austa SK | Latvia | 36 | KC Virtus | Hungary |
| 5 | Balaton | Hungary | 37 | KC Zuzana | Hungary |
| 6 | Beniko | Switzerland | 38 | KCA Borana | Hungary |
| Czech Republic | |||||
| Nederland | |||||
| Poland | |||||
| 7 | Bialobrzeskie | Czech Republic Polan |
39 | Kompoliti | Hungary |
| 8 | Cannakomp | Hungary | 40 | Kompoliti Hibrid TC | Hungary |
| 9 | Carma | Italy | 41 | Lipko | Hungary |
| 10 | Carmaleonte | Italy | 42 | Lovrin 110 | Romania |
| 11 | Chamaeleon | Nederland | 43 | Marcello | Nederland |
| 12 | Codimono | Italy | 44 | Markant | Nederland |
| 13 | Dacia Secuieni | Romania | 45 | Monoica | Switzerland |
| Hungary | |||||
| 14 | Delta-405 | Spanish | 46 | Orion 33 | France |
| 15 | Delta-Ilosa | Spanish | 47 | Rajan | Poland |
| 16 | Dioica 88 | France | 48 | Ratza | Romania |
| 17 | Earlina 8 FC | France | 49 | Santhica 23 | France |
| 18 | Eletta Campana | Italy | 50 | Santhica 27 | France |
| 19 | Epsilon 68 | France | 51 | Santhica 70 | France |
| 20 | Fedora 17 | Switzerland | 52 | Secuieni Jubileu | Romania |
| France | |||||
| 21 | Felina 32 | France | 53 | Silvana | Romania |
| 22 | Fibrante | Italy | 54 | Succesiv | Romania |
| 23 | Fibrol | Hungary | 55 | Teodora | Romania |
| 24 | Fibror 79 | France | 56 | Tiborszallasi | HungaryItaly |
| 25 | Finola | Finland | 57 | Tisza | Hungary |
| 26 | Futura 75 | France | 58 | Tygra | Poland |
| 27 | Futura 83 | France | 59 | Uniko B | Hungary |
| 28 | Fèrimon | Deutschland France |
60 | Uso-31 | Nederland |
| 29 | Glecia | Italy | 61 | Villanova | Italy |
| 30 | Gilana | Italy | 62 | Wielkopolskie | Poland |
| 31 | Glyana | Poland | 63 | Wojko | Poland |
| 32 | Henola | Poland | 64 | Zenit | Romania |
Nowadays the EU is the world’s largest hemp-producing market second only to Canada, with France, the Netherlands, Lithuania, and Romania as the major production centres [31][31]. According to the EU guidelines, also in Italy, the cultivation of industrial hemp has been recently restored through the law n. 242/2016, and the subsequent circular of the Ministry of Agricultural, Food and Forestry Policies (MIPAAF) published on 14 January 2017 that has delineated the conditions for hemp production, its commercialization, and its utilization for specific industrial purposes [38][38].
Finally, in the US, the federal policy regarding hemp was significantly altered with the 2014 Farm Bill (Agricultural Act of 2014) that allowed the USDA and certain research institutions to grow hemp under an agricultural pilot program. Despite this act, industrial hemp continued to be a niche crop. The great novelty was four years later with the 2018 Farm Bill that has established a new federal hemp regulatory system under the USDA with the aim to facilitate the commercial cultivation, processing, and marketing of hemp and to essentially treat hemp like any other agricultural commodity. Indeed, it removed hemp (i.e., C. sativa L. varieties with a THC content <0.3% of the dry weight of leaves and flowering parts) and their products—among which is hempseed—from the statutory definition of the drug marijuana and the DEA schedule of controlled substances, opening the hemp industry for business [31][31]. 2018 was also the year in which the federal government of Canada legalized access to recreational cannabis (i.e., the drug-type C. sativa L.) through the entry into force of the Cannabis Act, Bill C-45 [40][40]. In Figure 3, the main highlights about the C. sativa L. legislation in Canada, the US, the EU and Italy among the EU states, are illustrated.
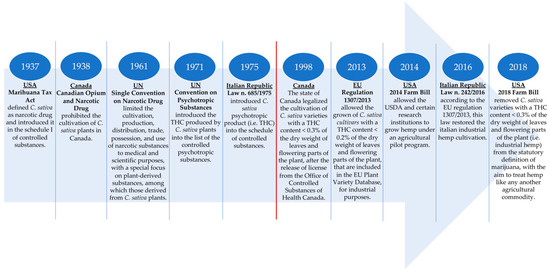
The main points of the legislation about
C. sativaL. (industrial hemp) cultivation in the US, Canada, and the EU with a focus on the Italian Republic among the EU states. The red line separates the legislation related to the prohibition of
C. sativa L. cultivation (left part of the red line) from that allowed the reintroduction of growing of this crop (right part of the red line). US: United States; UN: United Nations; THC: delta-9-tetrahydrocannabinol; EU: European Union; USDA: United Stated Department of Agricultural.L. cultivation (left part of the red line) from that allowed the reintroduction of growing of this crop (right part of the red line). US: United States; UN: United Nations; THC: delta-9-tetrahydrocannabinol; EU: European Union; USDA: United Stated Department of Agricultural.
References
- Ashutosh Mukherjee; Satyesh Chandra Roy; S. De Bera; Hong-En Jiang; Xiao Li; Cheng-Sen Li; Subir Bera; Results of molecular analysis of an archaeological hemp (Cannabis sativa L.) DNA sample from North West China. Genetic Resources and Crop Evolution 2008, 55, 481-485, 10.1007/s10722-008-9343-9.
- John M. McPartland; Geoffrey W. Guy; William Hegman; Cannabis is indigenous to Europe and cultivation began during the Copper or Bronze age: a probabilistic synthesis of fossil pollen studies. Vegetation History and Archaeobotany 2018, 27, 635-648, 10.1007/s00334-018-0678-7.
- Maria Irakli; Eleni Tsaliki; Apostolos Kalivas; Fotios Kleisiaris; Eirini Sarrou; Catherine M Cook; Effect οf Genotype and Growing Year on the Nutritional, Phytochemical, and Antioxidant Properties of Industrial Hemp (Cannabis sativa L.) Seeds.. Antioxidants 2019, 8, 491, 10.3390/antiox8100491.
- European Monitoring Centre for Drugs and Drug Addiction (EMCDDA). Cannabis Legislation in Europe: An Overview; Publications Office of the European Union: Luxembourg, 2018.
- M. David Marks; Li Tian; Jonathan P. Wenger; Stephanie N. Omburo; Wilfredo Soto-Fuentes; Ji He; David R. Gang; George D. Weiblen; Richard A. Dixon; Identification of candidate genes affecting Delta9-tetrahydrocannabinol biosynthesis in Cannabis sativa.. Journal of Experimental Botany 2009, 60, 3715-3726, 10.1093/jxb/erp210.
- Supaart Sirikantaramas; Futoshi Taura; Yumi Tanaka; Yu Ishikawa; Satoshi Morimoto; Yukihiro Shoyama; Tetrahydrocannabinolic Acid Synthase, the Enzyme Controlling Marijuana Psychoactivity, is Secreted into the Storage Cavity of the Glandular Trichomes. Plant and Cell Physiology 2005, 46, 1578-1582, 10.1093/pcp/pci166.
- Chiara Onofri; Etienne P.M. De Meijer; Giuseppe Mandolino; Sequence heterogeneity of cannabidiolic- and tetrahydrocannabinolic acid-synthase in Cannabis sativa L. and its relationship with chemical phenotype. Phytochemistry 2015, 116, 57-68, 10.1016/j.phytochem.2015.03.006.
- Christina Staginnus; Siegfried Zörntlein; Etienne De Meijer; A PCR marker Linked to a THCA synthase Polymorphism is a Reliable Tool to Discriminate Potentially THC-Rich Plants of Cannabis sativa L.. Journal of Forensic Sciences 2014, 59, 919-926, 10.1111/1556-4029.12448.
- Cinzia Citti; Barbara Pacchetti; M.A. Vandelli; Flavio Forni; Giuseppe Cannazza; Analysis of cannabinoids in commercial hemp seed oil and decarboxylation kinetics studies of cannabidiolic acid (CBDA). Journal of Pharmaceutical and Biomedical Analysis 2018, 149, 532-540, 10.1016/j.jpba.2017.11.044.
- Úrsula Escrivá; María Jesús Andrés-Costa; Vicente Andreu; Yolanda Picó; Analysis of cannabinoids by liquid chromatography–mass spectrometry in milk, liver and hemp seed to ensure food safety. Food Chemistry 2017, 228, 177-185, 10.1016/j.foodchem.2017.01.128.
- Qingfang Meng; Beth Buchanan; Jonathan Zuccolo; Mathieu-Marc Poulin; Joseph Gabriele; David Charles Baranowski; A reliable and validated LC-MS/MS method for the simultaneous quantification of 4 cannabinoids in 40 consumer products. PLOS ONE 2018, 13, e0196396, 10.1371/journal.pone.0196396.
- Cinzia Citti; Pasquale Linciano; Sara Panseri; Francesca Vezzalini; Flavio Forni; Maria Angela Vandelli; Giuseppe Cannazza; Cannabinoid Profiling of Hemp Seed Oil by Liquid Chromatography Coupled to High-Resolution Mass Spectrometry. Frontiers in Plant Science 2019, 10, 120, 10.3389/fpls.2019.00120.
- Christinat, N.; Savoy, M.-C.; Mottier, P.; Development, validation and application of a LC-MS/MS method for quantification of 15 cannabinoids in food. Food Chem. 2020, 318, 126469, 10.1016/j.foodchem.2020.126469.
- Monika Fellermeier; Meinhart H. Zenk; Prenylation of olivetolate by a hemp transferase yields cannabigerolic acid, the precursor of tetrahydrocannabinol.. FEBS Letters 1998, 427, 283-285, 10.1016/s0014-5793(98)00450-5.
- Monika Fellermeier; Wolfgang Eisenreich; Adelbert Bacher; Meinhart H. Zenk; Biosynthesis of cannabinoids. Incorporation experiments with (13)C-labeled glucoses.. JBIC Journal of Biological Inorganic Chemistry 2001, 268, 1596-1604, 10.1046/j.1432-1033.2001.02030.x.
- Christian Giroud; Analysis of Cannabinoids in Hemp Plants. CHIMIA International Journal for Chemistry 2002, 56, 80-83, 10.2533/000942902777680702.
- Futoshi Taura; Shinji Tanaka; Chiho Taguchi; Tomohide Fukamizu; Hiroyuki Tanaka; Yukihiro Shoyama; Satoshi Morimoto; Characterization of olivetol synthase, a polyketide synthase putatively involved in cannabinoid biosynthetic pathway. FEBS Letters 2009, 583, 2061-2066, 10.1016/j.febslet.2009.05.024.
- Steve J. Gagne; Jake Stout; Enwu Liu; Zakia Boubakir; Shawn M. Clark; Jonathan E. Page; Identification of olivetolic acid cyclase from Cannabis sativa reveals a unique catalytic route to plant polyketides. Proceedings of the National Academy of Sciences 2012, 109, 12811-12816, 10.1073/pnas.1200330109.
- Judith K. Booth; Jonathan E. Page; Joerg Bohlmann; Terpene synthases from Cannabis sativa. PLOS ONE 2017, 12, e0173911, 10.1371/journal.pone.0173911.
- Daniela Pacifico; Francesca Miselli; Mirta Micheler; Andrea Carboni; Paolo Ranalli; Giuseppe Mandolino; Genetics and Marker-assisted Selection of the Chemotype in Cannabis sativa L.. Molecular Breeding 2006, 17, 257-268, 10.1007/s11032-005-5681-x.
- D. Rotherham; Sa Harbison; Differentiation of drug and non-drug Cannabis using a single nucleotide polymorphism (SNP) assay. Forensic Science International 2011, 207, 193-197, 10.1016/j.forsciint.2010.10.006.
- Julian Broséus; Frédéric Anglada; Pierre Esseiva; The differentiation of fibre- and drug type Cannabis seedlings by gas chromatography/mass spectrometry and chemometric tools. Forensic Science International 2010, 200, 87-92, 10.1016/j.forsciint.2010.03.034.
- Etienne P M De Meijer; Manuela Bagatta; Andrea Carboni; Paola Crucitti; V M Cristiana Moliterni; Paolo Ranalli; Giuseppe Mandolino; The inheritance of chemical phenotype in Cannabis sativa L.. Genetics 2003, 163, 335-346.
- Fidelia Cascini; Alessio Farcomeni; Daniele Migliorini; Laura Baldassarri; Ilaria Boschi; Simona Martello; Stefano Amaducci; Luigi Lucini; Jamila Bernardi; Highly Predictive Genetic Markers Distinguish Drug-Type from Fiber-Type Cannabis sativa L.. Plants 2019, 8, 496, 10.3390/plants8110496.
- Mareshige Kojoma; Hikaru Seki; Shigeo Yoshida; Toshiya Muranaka; DNA polymorphisms in the tetrahydrocannabinolic acid (THCA) synthase gene in “drug-type” and “fiber-type” Cannabis sativa L.. Forensic Science International 2006, 159, 132-140, 10.1016/j.forsciint.2005.07.005.
- De Meijer, E. The Chemical Phenotypes (Chemotypes) of Cannabis, 1st ed.; Handbooks in Psychopharmacology; Oxford University Press: Oxford, UK; New York, NY, USA, 2014; ISBN 978-0-19-966268-5.
- Karl W. Hillig; Genetic evidence for speciation in Cannabis (Cannabaceae). Genetic Resources and Crop Evolution 2005, 52, 161-180, 10.1007/s10722-003-4452-y.
- Steve G. U. Naraine; Ernest Small; Andrew E. Laursen; Lesley G. Campbell; A multivariate analysis of morphological divergence of “seeds” (achenes) among ruderal, fibre, oilseed, dioecious/monoecious and marijuana variants of Cannabis sativa L.. Genetic Resources and Crop Evolution 2019, 67, 703-714, 10.1007/s10722-019-00848-9.
- John M. McPartland; CannabisSystematics at the Levels of Family, Genus, and Species. Cannabis and Cannabinoid Research 2018, 3, 203-212, 10.1089/can.2018.0039.
- Ernest Small; Arthur Cronquist; A PRACTICAL AND NATURAL TAXONOMY FOR CANNABIS. TAXON 1976, 25, 405-435, 10.2307/1220524.
- Johnson, R. Hemp as an Agricultural Commodity; CRS Report RL32725; Congressional Research Service: Washington, DC, USA, 2018; Available online: https://fas.org/sgp/crs/misc/RL32725.pdf (accessed on 12 March 2020).
- Carla Da Porto; Deborah Decorti; Andrea Natolino; Potential Oil Yield, Fatty Acid Composition, and Oxidation Stability of the Hempseed Oil from FourCannabis sativaL. Cultivars. Journal of Dietary Supplements 2014, 12, 1-10, 10.3109/19390211.2014.887601.
- Cecil L. Vera; Arthur Hanks; Hemp Production in Western Canada. Journal of Industrial Hemp 2004, 9, 79-86, 10.1300/j237v09n02_08.
- Eliana Vonapartis; Marie-Pier Aubin; Philippe Seguin; Arif F. Mustafa; Jean-Benoit Charron; Seed composition of ten industrial hemp cultivars approved for production in Canada. Journal of Food Composition and Analysis 2015, 39, 8-12, 10.1016/j.jfca.2014.11.004.
- Sabet, K.A. Single Convention on Narcotic Drugs, 1961. In Encyclopedia of Drug Policy; SAGE Publications, Inc.: Thousand Oaks, CA, USA, 2011; ISBN 978-1-4129-7695-4.
- United Nation Convention on Psychotropic Substances. Available online: https://www.unodc.org/unodc/en/treaties/psychotropics.html (accessed on 12 March 2020)
- European Commission EU Plant Variety Database (v.3.2). Available online: https://ec.europa.eu/food/plant/plant_propagation_material/plant_variety_catalogues_databases/search/public/index.cfm?event=SearchVariety&ctl_type=A&species_id=240&variety_name=&listed_in=0&show_current=on&show_deleted= (accessed on 15 March 2020).
- Radmila Pavlovic; Sara Panseri; Luca Giupponi; Valeria Leoni; Cinzia Citti; Chiara Cattaneo; Maria Cavaletto; Annamaria Giorgi; Phytochemical and Ecological Analysis of Two Varieties of Hemp (Cannabis sativa L.) Grown in a Mountain Environment of Italian Alps.. Frontiers in Plant Science 2019, 10, 1265, 10.3389/fpls.2019.01265.
- Incoronata Galasso; Roberto Russo; Sergio Mapelli; Elena Ponzoni; Ida M. Brambilla; Giovanna Battelli; Remo Reggiani; Variability in Seed Traits in a Collection of Cannabis sativa L. Genotypes. Frontiers in Plant Science 2016, 7, 65, 10.3389/fpls.2016.00688.
- Senate of Canada—The Cannabis Act in the Senate. Available online: https://www.sencanada.ca/en/sencaplus/news/cannabis-act/ (accessed on 15 January 2020).
