Film-forming formulations represent a novel form of sustained release dermatic products. They are applied to the skin as a liquid or semi-solid preparation.
1. Introduction
1.1. Skin
The skin is the largest organ of the human body
[1]. It acts as a protective barrier against external influences such as ultraviolet radiation, chemical and physical insults, attacks by harmful microorganisms, and mechanical irritation. Furthermore, the skin regulates physiological parameters of the body by providing a barrier against water evaporation and temperature loss
[2].
Skin diseases such as infections triggered by bacteria or viruses, as well as the immunologically caused chronic skin diseases psoriasis, atopic dermatitis, urticaria, or ichthyosis damage the skin barrier. Physiological functions can no longer be maintained, increasing the risk of further infection. Symptoms such as pain, soreness, and wetness affect the patients’ quality of life
[3].
1.2. Transdermal Transport
Penetration into the stratum corneum is the limiting factor for the amount of drug that can be absorbed
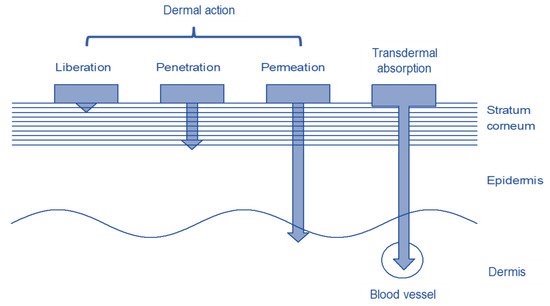
[9][4]. Drugs can pass through the stratum corneum by trans epidermal, trans follicular, or trans glandular routes. Depending on the drug and formulation, they penetrate the skin to different depths as shown in
Figure 1 [10][5]. Whether and how much of a substance penetrates the skin depends on several factors. The properties of the substance to be applied to the skin and the formulation in which it is integrated have the greatest influence on penetration. The condition of the skin, i.e., the skin area and the condition of the skin barrier, which in turn depend on age and individual living conditions, also influences penetration
[2].
Figure 1.
Structure of the skin with the different penetration depths of the dermal and transdermal systems.
Three different penetration mechanisms can be distinguished in the penetration of drugs via trans epidermal route. In the transcellular pathway, the drug alternately penetrates the hydrophilic intracellular space, the corneocytes with their protein envelope and the corneodesmosomes, and the lipophilic intercellular space, the lipophilic matrix. This multiple portioning between hydrophilic and lipophilic matrix is very unlikely.
Via the intercellular route, drugs penetrate by crossing the stratum corneum through the lipophilic matrix. Substances that have a predominantly lipophilic character usually penetrate quickly and completely into the lipophilic matrix. As the underlying layers of the dermis are of a less lipophilic character, the penetration of highly lipophilic substances from the stratum corneum into these skin layers is usually slow, so that a reservoir of the substance is formed in the stratum corneum
[11][6]. Drugs with a dominating hydrophilic character can also penetrate via the intercellular route along the hydrated hydrophilic head groups of the stratum corneum lipids.
In addition to the trans epidermal route, lipophilic drugs in particular penetrate well into deeper skin layers via the trans follicular route. Penetration is rapid as, unlike the trans epidermal route, fewer cell layers must be overcome. However, the amount of active ingredient that penetrates is strongly dependent on the amount and character of the hair glands, so this route depends strongly on individual factors
[14][7].
The glandular route can be neglected, as the majority of the drug is transported out wards by secretion which means that this route cannot be considered for reliable penetration. The penetration pathways described have been researched using healthy skin. In the case of chronic skin diseases such as psoriasis, atopic dermatitis, chronic dermatitis, and ichthyosis, this skin barrier is disturbed, which makes the development of formulations for therapy challenging. The penetration of substances cannot be adequately predicted.
1.3. Dermal Systems
Dermal systems are usually designed to only achieve a local effect. They are used for the therapy of acute skin infections and chronic skin diseases. Usually, a liquid or semi-solid formulation is applied at regular intervals. The formulation should be designed in such a way that the active ingredient contained can penetrate into the epidermis and develop its effect. To avoid a systemic effect, penetration into the dermis should be kept to a minimum. Especially in the case of drugs such as antibiotics and immunosuppressants such as cortisone, a sufficiently high therapeutic quantity can be achieved by dermal application without systemically burdening the organism with the drugs. The risk-benefit profile can be influenced positively.
Conventional topical preparations are easily washed or rubbed off. Thus, the treatment is often inadequate as the active ingredient is not present in the epidermis in sufficient and consistent quantities. Using a higher concentration of the drug is not a solution for this drawback, as the concentration fluctuations would only be amplified and the probability of active substance penetrating into the dermis would increase. Formulations exhibiting high substantivity provide a more elegant solution. The substantivity is defined as the capacity of the active substance to be kept at the site of action, on the surface or as a reservoir in the stratum corneum.
Using patches and plasters for dermal treatment of skin diseases may provide an alternative. They create a physical barrier between the formulation and the environment which protects the formulation from erosion. This physical barrier usually leads to occlusion of the skin, which, in addition to the affections of unappealing appearance and negative wearing comfort, is detrimental to patient compliance. In most cases, the patches cannot be cut to the required size, which often makes them unsuitable for individualised therapy. Skin damage can occur when the patches are pulled off, which makes them unsuitable for use in diseases with a disturbed skin barrier. Allergic reactions to the matrix adhesives are another problem, so these systems are not optimal either
[15][8].
1.4. Transdermal Systems
In transdermal systems, an active ingredient incorporated into a liquid or semi-solid formulation is applied to the skin, or the drug is delivered via a patch. In contrast to dermal systems, the aim is to deliver the active to the systemic circulation. Compared to other forms of delivery, transdermal systems offer many advantages. They are one way to avoid first pass effect. Food effects or intestinal absorption problems are no issue. Compared to injections or intravenous applications during the administration of drugs, the skin is not injured by needles or incisions
[16][9].
With patches, which are the most common treatment, the sustained release of the drug is usually easy to realise. The drug is embedded in its formulation in the membrane of the patch and may be released through a control membrane or is controlled by diffusion in the adhesive. Patches are usually easy to apply, as they are simply administered at correct time intervals to uninjured, hairless areas of the body surface. When properly applied, the drug release is continuous so that the plasma level is constant. The active ingredient is well protected from external influences in the formulation by the backing layer. In the case of insufficient penetration, in addition to penetration enhancers, systems such as microneedles or iontophoresis can also be used to improve penetration. The disadvantages of patches for transdermal use are the same as in the use of dermal delivery, namely the cosmetic aspect. Currently, mainly opiates and hormones are applied transdermally. However, transdermal application is an option for a lot of drugs with a low therapeutic range and drugs with stability problems
[17][10].
2. Film-Forming Systems
As described, the main problem with dermal and transdermal application of drugs via liquid and semi-solid formulations is the washing and rubbing off of the formulations, so that the desired therapeutic effect cannot be achieved. For this reason, very few semi-solid formulations are found in transdermal use. The idea behind the development of film-forming formulations is to develop formulations with increased substantivity against mechanical and water-based influences and improvement of the cosmetic properties, and thus patients’ compliance. As dosage forms, sprays, gels, or emulsions may be formulated
[18,19][11][12].
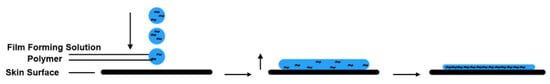
As shown in
Figure 2, the formulation is applied evenly to the skin surface. After the application of the solution or gel, the volatile solvent evaporates rapidly and the film-forming polymers interact with one another to form a thin transparent film, which adheres to the skin
[20][13].
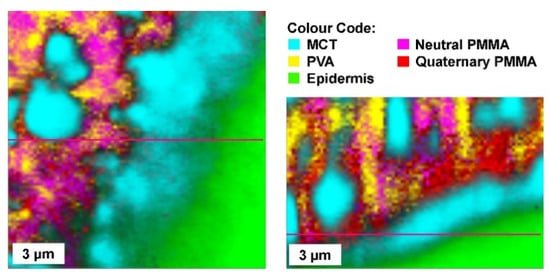
Figure 3 shows Raman-microscopic images of a film-forming emulsion applied to the skin. The emulsion is applied in a liquid state and forms a solid film after drying, similar to the film-forming solutions and gels.
Figure 2.
Mechanism of film forming.
Figure 3.
Film-forming emulsion, the oil droplets are embedded in a polymer matrix made of PVA and Eudragit NE (neutral PMMA (Polymethylmethacrylate) and Eudragit RS (quarternatry PMMA) for controlled drug release.
In addition to the volatile vehicle and the film-forming agent, the film-forming systems contain the active ingredient and usually a plasticiser and/or penetration enhancer. These non-volatile components ensure that the dry film does not have any brittle properties but adheres to the skin and forms a flexible and non-tacky film after the evaporation of the volatile solvent. The resulting film must be such that it forms a homogenic reservoir of the active ingredient on the skin which has a high substantivity, from which the active ingredient can still penetrate well into the stratum corneum
[21][14]. Due to enhanced substantivity, the formulation can also be used as a transdermal system as the correct dose can be delivered reliably. The formed film must adhere firmly to the skin and must not wash or rub off. The drug can have its reservoir in the formulation itself, and penetrate slowly into the stratum corneum, or pass directly from the formulation into the stratum corneum and form its reservoir there. In the latter case, the formed film serves as protection.
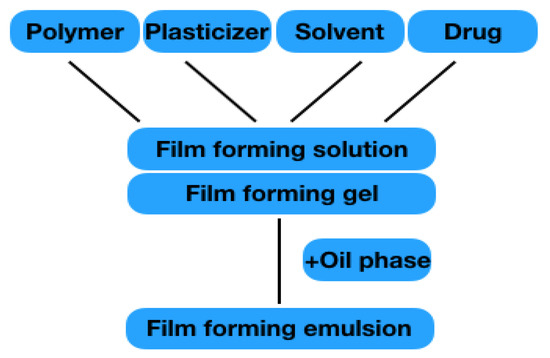
In the field of semi-solid preparations, gels are listed as film-forming formulations. In the area of liquid preparations, emulsions and solutions, as well as so-called patch-no-patch systems, are discussed. Nano-formulations or formulation for wound healing are not addressed, as dedicated reviews on these topics exist. All formulations mainly consist of the components listed in 2.1.1–2.1.4. The structure is shown in Figure 4. The film formation of the individual formulation results from the polymers it contains. When the solvent evaporates, the polymer chains interact with one another, and are able to form a solid polymer matrix on the skin surface with a high substantivity that forms a reservoir for the drug. The drug penetrates from the film into the skin to exert its effects locally or systemically.
Figure 4.
Illustration of different film-forming solution/gel.
2.1 Polymer
The choice of film-forming polymer has the greatest influence on the substantivity of the formulation. Polymers can be used individually or in combination. It is important that they are able to form a flexible, thin, transparent and resistant film. Essentially, a distinction is made between water-soluble and water-insoluble film formers [1]. Water soluble polymers have a hydrophilic character, and most of them are not suitable for substantivity increase in conventional formulations on the skin surface, but ideal for formulations from which the drug quickly penetrates the stratum corneum to form a drug reservoir there. Some, such as methylcellulose, are suitable to increase substantivity of thermo-emulsions by thermo gelation. In film-forming formulations, the aim is to create a drug reservoir in the formulation itself, for which the hydrophilic polymers alone are not suitable.
Water-insoluble polymers form water-resistant films with high substantivity, but are often brittle and inflexible, which makes adhesion to the skin difficult and causes the film-forming formulations to crumble. To increase the uniformity of the film and its flexibility, plasticisers are usually added to the formulation, or the polymer is combined with a water-soluble polymer [2]. Small polymers with a low molecular weight are usually better suited to film-forming systems. The viscosity of the formulation increases during the solvent evaporation process, even more so for polymers with a high molecular weight. Furthermore, smaller polymers with shorter chain lengths can usually arrange themselves better in space, so that the distance for the interaction of the polymer chains is closer to the ideal state for gel formation. After the evaporation of the volatile solvent, the formed film adheres uniformly to the skin, so that no major differences in film thickness result. This is particularly important for the penetration of the active ingredient into the skin, as the concentration gradient between the film and the stratum corneum is thus also constant over a longer period of time on all areas of the skin [3].
Polymers with different properties that have been used for preparation of film-forming systems are mentioned in Table 1. In the future, environmental influences will also become increasingly relevant in the choice of film formers, as most insoluble film formers for modified active ingredient release are considered microplastics, which enter wastewater through precipitation, abrasion, and washing. Therefore, starch, alginate, or cellulose-based formulations should be the focus of the development process in the future [415].
Table 1.
Polymers for use in film-forming formulations.
|
Polymer
|
Properties
|
|
Polymer
|
Properties
|
|
Carbopol (polyacrylat)
|
water-soluble, pH sensitive
|
|
Chitosan (Poly-D-Glucosamin)
|
water-soluble at pH < 7
|
|
Crosslinked polymer layer XPL
|
adhesive, elastic
|
|
Dermacryl 79 (Carboxylates Ac
rylpolymer)
|
water-insoluble
|
|
Ethylcellulose
|
non-toxic, not irritating, anti-allergic
|
|
Eudragit NE (ethylacrylate methylmethacrylate copolymer)
|
water-insoluble, transparent, elastic, adhesive
|
|
Eudragit RL-100 (polymethacrylate polymere)
|
water-insoluble, transparent, elastic, adhesive
|
|
Eudragit RS-100 (polymethacrylate polymere)
|
water-insoluble, transparent, elastic, adhesive
|
|
Eudragit L30D-55 (methacrylate-ethylacrylate-copolymer)
|
water dispersible at pH 2–3
|
|
Hydroxypropyl-beta-cyclodextrin
|
water-insoluble, increases bioavailability
|
|
Hydroxypropylmethylcellulose (HPMC)
|
water-soluble, non-ionic
|
|
KIucel (Hydroxypropyl cellulose)
|
water-soluble, non-ionic
|
|
Macrogol
|
water-soluble
|
|
Methyl cellulose
|
water-soluble
|
|
Poloxamer (polyethylenepolypropylene glycol)
|
thermoreversible
|
|
Plastoid (Butyl methacrylate-methylmethacrylate copolymer)
|
water-insoluble
|
|
Polydimethylsiloxane (PDMS)
|
water-insoluble, non-toxic
|
|
Polyvinyl alcohol (PVA)
|
water-soluble, adhesive, non-toxic
|
|
Polyvinyl pyrrolidine (PVP)
|
water-soluble, adhesive, increase bioavailability
|
|
Quaternary polymethacrylat (QPM)
|
water-insoluble
|
|
Sepineo P600 (acrylamide/sodium acryloldimethyltaurate)
|
water-insoluble
|
|
Silicone
|
water-soluble, non-occlusive
|
3. Development and Investigation of Film-Forming Systems
Various groups are working on the development of film-forming formulations with different drugs. The topic is especially interesting for the development of sustained-release dermal products. In recent years, various excipients have been compared and tested for their suitability. The development and evaluation of suitable test systems for the comparison of film-forming formulations is indispensable for their development.
The properties of the formulations with regard to drying time, film thickness, film weight, rheological studies, adherence and spreading, stickiness, water vapor permeability, mechanical properties, and film homogeneity must be suitable and compared with one another. The developed formulations should be free of toxic or allergenic ingredients
[56][16]. In case of dermal and transdermal systems, the penetration and permeation of the systems must be investigated
[35][17]. Today, only a few film-forming formulations are on the market. For many drugs for which a dermal or transdermal application option would be useful, film-forming formulations are not yet available. A large number of polymers are still available for the development of new systems. Film-forming systems that have been developed recently will be described in the following chapters.
4. Conclusions and Further Prospects
According to previous research findings, film-forming systems have proven to be suitable for use as a delivery form due to their high substantivity and cosmetic attractiveness. Drugs can penetrate from the systems into the skin, which enables dermal and transdermal drug application. The formulations also serve as a depot, which enables a sustained release. While the use of film-forming sprays in the field of wound care is already established and well accepted by patients, film-forming systems are still the exception in the treatment of diseases.
In the field of transdermal application, there is, with the testosterone spray Axiron
®, already one formulation on the market. For transdermal use, film-forming systems have not yet been able to establish themselves as a therapeutic option against other transdermal systems such as patches. For dermal application, only film-forming solutions for the treatment of topical infections, such as the terbinafine Lamisil Once
® spray, have made it to the market; while studies on the treatment of chronic skin diseases were carried out, but no formulation is yet available for patients, neither for daily therapy in the form of cortisone preparations, nor as NSAIDs for acute therapy. The development of therapies for chronic inflammatory skin diseases and topical infections with film-forming formulations could be pursued more vigorously in the future.
Film-forming solutions that are sprayed directly onto the skin create the sensation of a second skin, unlike patch-no-patch systems. On the other hand, precise application and dosing are easier to implement by using patch-no-patch, which are also able to protect the treated skin area and show increased physicochemical stability. Film-forming emulsions also contain a lipophilic phase and can therefore also be used on dry skin areas. Due to their semi-solid character, gels have an advantage in that that they are easier and more precise to apply. All systems are well suited for personalised therapy. The semi-solid and liquid preparations can be produced in individual concentrations and the films can be printed individually.
Furthermore, there are comparatively few developments of film-forming formulations of drugs with hydrophilic character. A focus on the development of such formulations could provide access to further treatment fields.
The formulations can reach out more to the optimal condition of a second skin by optimising the concentration of the individual components and using new ingredients, thus increasing patient compliance. In this context, gels and emulsions can also become more important than the film-forming sprays that have been predominantly established to date, as they are easier to apply.
Another future field of research is the development of lipophilic film-forming systems with oil-soluble film-forming agents. These formulations could be used for chronic inflammatory skin diseases and, in addition to retarded release of the active ingredient and high substantivity, have positive effects on skin properties. This would allow daily therapy with drugs such as cortisone derivatives to be combined with basic or maintenance therapy.