There are four known stereoisomers of jasmonic acid: trans-(−)-(3R,7R), abbreviated as (−)-JA; trans-(+)-(3S,7S) abbreviated as (+)-JA; cis-(−)-(3S,7R) abbreviated as (−)-epi-JA; cis-(+)-(3R,7S) abbreviated as (+)-epi-JA [15]. The naturally occurring jasmonic acid in plants is (−)-JA and (+)-epi-JA. Due to the fact that the cis stereoisomers are thermodynamically less stable, they epimerize at the C-7 atom to the stable trans form, which at the same time shows a higher biological activity. The biological activity of jasmonic acid has been found to be dependent on the presence of a carboxyl group at the C-1 position, a keto or hydroxyl group at the C-6 position, and a pentenyl side chain at the C-7 position [16,17,18]. Because of this structure, jasmonates inhibit, induce and/or stimulate changes that occur in plants at the morphological, physiological, cellular and molecular levels.
- jasmonic acid
- anti-cancer drugs
- structure–activity relationship
1. Introduction
2. Jasmonic Acid and Its Derivatives Occurring in Plants
3. Chemical Structure of Jasmonates
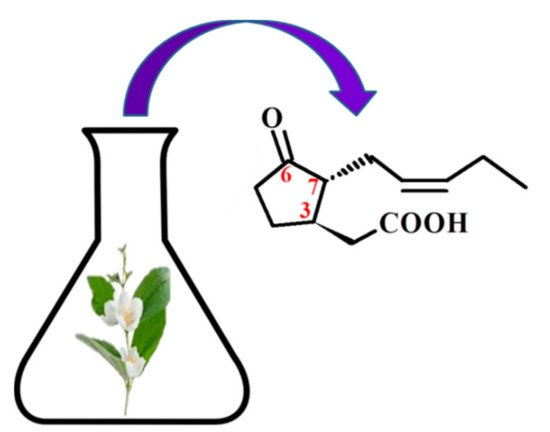
4. Pharmacological Activity of Jasmonates
5. Therapeutic Benefits of Combining Jasmonates with Anti-Cancer Drugs
| Jasmonates/Drug | Cancer | Concentration Range | Action/Effects | References | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MJ + BCNU | (in vitro) | MJ + taxol | (in vitro) | Pancreatic cell | : PaCa-2 BCL1 MCF-7 DA-3 D-122 |
MJ: 0.1 mM BCNU: 1, 10, 25 µg/mL-PaCa-2 2.5, 5 µg/mL-BCL1 taxol: 1, 2.5, 5, 10 μg/ml |
mitochondriotoxic synergic cytotoxicity IC | 50 | ↓ | [54] | [35] | |||||||
| MJ + POH | or | MJ + cisplatin | or | MJ + cisplatin + POH | (in vitro) | Breast cancer cell lines | : MDA-MB-231 MDA-MB-435 MCF7 |
IC | 2O | (POH) MDA-MB-231: 0.76 mM MDA-MB-435: 0.6 mM MCF7: 0.8 mM |
MJ + POH: cytotoxicity ↑ apoptosis ↑ TNFR1 ↑ MJ + POH: apoptosis ↑ |
[55] | [36] | |||||
| MJ + 2DG | 2-deoxyglucose | (in vitro) | Sarcoma | : SaOS-2 MCA-105 |
MJ: 0.5–3 mM 2DG: 1 and 2 mM |
synergic cytotoxicity ↑ ATP glycolysis ↑ |
[56] | [37] | ||||||||||
| MJ + TRAIL | (in vitro) | CRC cell lines: SW480, HT29, LS180, HCT116 |
MJ: 0.5 mM TRAIL: 100–200 ng/ml |
IAP (survivin) ↓ caspase activity ↑ TRAIL-induced apoptosis ↑ |
[58] | [38] | ||||||||||||
| MJ + Smac7N | (in vitro) | prostate carcinoma cells | : DU145, PC-3 proximal tubular epithelial cells: HK-2 |
MJ: 0.5–2 mM | Smac7N: MJ-induced cytotoxicity ↑ ran caspase-9 dependent and independent pathways |
[60] | [39] | |||||||||||
| MJ + 3-BrP | (in vitro) | Mice breast carcinoma cell line | : 4 T1 |
MJ: 0.5–3 mM 3-BrP: 12.5, 25, 50, 100, 200, 400 μM |
ALT ↑ AST ↑ tumor growth ↓ antitumor activity ↑ |
[53] | [40] | |||||||||||
| MJ + cisplatin | MJ + X-rays | MJ + α-rays | Cervical cancer cells | : SiHa, CaSki, HeLa C33A |
MJ: 0.1–1 mM Cisplatin: 0.1–0.5 μM X-rays: 0 25–3 Gy |
cell survival ↓ IC | 50 | radiation dose ↓ cell viability ↓ |
[62] | [41] |
References
- Choudhary, D.; Goykar, H.; Karanwad, T.; Kannaujia, S.; Gadekar, V.; Misra, M. An Understanding of Mitochondria and Its Role in Targeting Nanocarriers for Diagnosis and Treatment of Cancer. Asian J. Pharm. Sci. 2020.
- Kuruppu, A.I.; Paranagama, P.; Goonasekara, C.L. Medicinal Plants Commonly Used against Cancer in Traditional Medicine Formulae in Sri Lanka. Saudi Pharm. J. SPJ Off. Publ. Saudi Pharm. Soc. 2019, 27, 565–573.
- Koklesova, L.; Liskova, A.; Samec, M.; Qaradakhi, T.; Zulli, A.; Smejkal, K.; Kajo, K.; Jakubikova, J.; Behzadi, P.; Pec, M.; et al. Genoprotective Activities of Plant Natural Substances in Cancer and Chemopreventive Strategies in the Context of 3P Medicine. EPMA J. 2020, 11, 261–287.
- Wilmowicz, E.; Frankowski, K.; Sidłowska, M.; Kućko, A.; Kesy, J.; Gasiorowski, A.; Glazińska, P.; Kopcewicz, J. [Jasmonate biosynthesis—The latest discoveries]. Postepy Biochem. 2012, 58, 26–33.
- Ghasemi Pirbalouti, A.; Sajjadi, S.E.; Parang, K. A Review (Research and Patents) on Jasmonic Acid and Its Derivatives. Arch. Pharm. 2014, 347, 229–239.
- Umukoro, S.; Alabi, A.O.; Eduviere, A.T.; Ajayi, A.M.; Oluwole, O.G. Anti-Inflammatory and Membrane Stabilizing Properties of Methyl Jasmonate in Rats. Chin. J. Nat. Med. 2017, 15, 202–209.
- Kramell, R.; Atzorn, R.; Schneider, G.; Miersch, O.; Brückner, C.; Schmidt, J.; Sembdner, G.; Parthier, B. Occurrence and Identification of Jasmonic Acid and Its Amino Acid Conjugates Induced by Osmotic Stress in Barley Leaf Tissue. J. Plant Growth Regul. 1995, 14, 29.
- Wasternack, C. Jasmonates: An Update on Biosynthesis, Signal Transduction and Action in Plant Stress Response, Growth and Development. Ann. Bot. 2007, 100, 681–697.
- Wang, J.; Song, L.; Gong, X.; Xu, J.; Li, M. Functions of Jasmonic Acid in Plant Regulation and Response to Abiotic Stress. Int. J. Mol. Sci. 2020, 21, 1446.
- Wasternack, C.; Kombrink, E. Jasmonates: Structural Requirements for Lipid-Derived Signals Active in Plant Stress Responses and Development. ACS Chem. Biol. 2010, 5, 63–77.
- Staswick, P.E.; Tiryaki, I. The Oxylipin Signal Jasmonic Acid Is Activated by an Enzyme That Conjugates It to Isoleucine in Arabidopsis. Plant Cell 2004, 16, 2117–2127.
- Suza, W.P.; Staswick, P.E. The Role of JAR1 in Jasmonoyl-L: -Isoleucine Production during Arabidopsis Wound Response. Planta 2008, 227, 1221–1232.
- Heitz, T.; Smirnova, E.; Widemann, E.; Aubert, Y.; Pinot, F.; Ménard, R. The Rise and Fall of Jasmonate Biological Activities. In Lipids in Plant and Algae Development; Subcellular Biochemistry; Nakamura, Y., Li-Beisson, Y., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 405–426. ISBN 978-3-319-25979-6.
- Swiatek, A.; Van Dongen, W.; Esmans, E.L.; Van Onckelen, H. Metabolic Fate of Jasmonates in Tobacco Bright Yellow-2 Cells. Plant Physiol. 2004, 135, 161–172.
- Koch, T.; Bandemer, K.; Boland, W. Biosynthesis of Cis-Jasmone: A Pathway for the Inactivation and the Disposal of the Plant Stress Hormone Jasmonic Acid to the Gas Phase? Helv. Chim. Acta 1997, 80, 838–850.
- Han, Y.; Bai, Y.; Xiao, Y.; Du, F.; Liang, Y.; Tan, Z.; Zhao, M.; Liu, H. Simultaneous Discrimination of Jasmonic Acid Stereoisomers by CE-QTOF-MS Employing the Partial Filling Technique. Electrophoresis 2011, 32, 2693–2699.
- Krumm, T.; Bandemer, K.; Boland, W. Induction of Volatile Biosynthesis in the Lima Bean (Phaseolus Lunatus) by Leucine- and Isoleucine Conjugates of 1-Oxo- and 1-Hydroxyindan-4-Carboxylic Acid: Evidence for Amino Acid Conjugates of Jasmonic Acid as Intermediates in the Octadecanoid Signalling Pathway. FEBS Lett. 1995, 377, 523–529.
- Koda, Y.; Kikuta, Y.; Tazaki, H.; Tsujino, Y.; Sakamura, S.; Yoshihara, T. Potato Tuber-Inducing Activities of Jasmonic Acid and Related Compounds. Phytochemistry 1991, 30, 1435–1438.
- Sá-Nakanishi, A.B.; Soni-Neto, J.; Moreira, L.S.; Gonçalves, G.A.; Silva, F.M.S.; Bracht, L.; Bersani-Amado, C.A.; Peralta, R.M.; Bracht, A.; Comar, J.F. Anti-Inflammatory and Antioxidant Actions of Methyl Jasmonate Are Associated with Metabolic Modifications in the Liver of Arthritic Rats. Oxid. Med. Cell. Longev. 2018, 2018, 2056250.
- Raviv, Z.; Cohen, S.; Reischer-Pelech, D. The Anti-Cancer Activities of Jasmonates. Cancer Chemother. Pharmacol. 2013, 71, 275–285.
- Fingrut, O.; Flescher, E. Plant Stress Hormones Suppress the Proliferation and Induce Apoptosis in Human Cancer Cells. Leukemia 2002, 16, 608–616.
- Cesari, I.M.; Carvalho, E.; Figueiredo Rodrigues, M.; Mendonça, B.D.S.; Amôedo, N.D.; Rumjanek, F.D. Methyl Jasmonate: Putative Mechanisms of Action on Cancer Cells Cycle, Metabolism, and Apoptosis. Int. J. Cell Biol. 2014, 2014, 572097.
- Pérez-Salamó, I.; Krasauskas, J.; Gates, S.; Díaz-Sánchez, K.E.; Devoto, A. An Update on Core Jasmonate Signalling Networks, Physiological Scenarios, and Health Applications. Annu. Plant Rev. 2019, 2.
- Kniazhanski, T.; Jackman, A.; Heyfets, A.; Gonen, P.; Flescher, E.; Sherman, L. Methyl Jasmonate Induces Cell Death with Mixed Characteristics of Apoptosis and Necrosis in Cervical Cancer Cells. Cancer Lett. 2008, 271, 34–46.
- Bömer, M.; Pérez-Salamó, I.; Florance, H.V.; Salmon, D.; Dudenhoffer, J.-H.; Finch, P.; Cinar, A.; Smirnoff, N.; Harvey, A.; Devoto, A. Jasmonates Induce Arabidopsis Bioactivities Selectively Inhibiting the Growth of Breast Cancer Cells through CDC6 and MTOR. New Phytol. 2021, 229, 2120–2134.
- Klippel, S.; Jakubikova, J.; Delmore, J.; Ooi, M.; McMillin, D.; Kastritis, E.; Laubach, J.; Richardson, P.G.; Anderson, K.C.; Mitsiades, C.S. Methyljasmonate Displays in vitro and in vivo Activity against Multiple Myeloma Cells. Br. J. Haematol. 2012, 159, 340–351.
- Fingrut, O.; Reischer, D.; Rotem, R.; Goldin, N.; Altboum, I.; Zan-Bar, I.; Flescher, E. Jasmonates Induce Nonapoptotic Death in High-Resistance Mutant P53-Expressing B-Lymphoma Cells. Br. J. Pharmacol. 2005, 146, 800–808.
- Kim, J.H.; Lee, S.Y.; Oh, S.Y.; Han, S.I.; Park, H.G.; Yoo, M.-A.; Kang, H.S. Methyl Jasmonate Induces Apoptosis through Induction of Bax/Bcl-XS and Activation of Caspase-3 via ROS Production in A549 Cells. Oncol. Rep. 2004, 12, 1233–1238.
- Kansara, S.; Parabia, F.M.; Badgujar, N.; Mistry, K. Effect of Methyl Jasmonate on A-549 Lung Cancer Cell Line and Docking with GLUT 1 and HK2 Targeted Proteins. Int. J. Cancer Tremnt. 2018, 1, 1–9.
- Ezekwudo, D.; Shashidharamurthy, R.; Devineni, D.; Bozeman, E.; Palaniappan, R.; Selvaraj, P. Inhibition of Expression of Anti-Apoptotic Protein Bcl-2 and Induction of Cell Death in Radioresistant Human Prostate Adenocarcinoma Cell Line (PC-3) by Methyl Jasmonate. Cancer Lett. 2008, 270, 277–285.
- Yeruva, L.; Elegbede, J.A.; Carper, S.W. Methyl Jasmonate Decreases Membrane Fluidity and Induces Apoptosis through Tumor Necrosis Factor Receptor 1 in Breast Cancer Cells. Anticancer. Drugs 2008, 19, 766–776.
- Tong, Q.-S.; Jiang, G.-S.; Zheng, L.-D.; Tang, S.-T.; Cai, J.-B.; Liu, Y.; Zeng, F.-Q.; Dong, J.-H. Methyl Jasmonate Downregulates Expression of Proliferating Cell Nuclear Antigen and Induces Apoptosis in Human Neuroblastoma Cell Lines. Anticancer. Drugs 2008, 19, 573–581.
- Zhang, M.; Su, L.; Xiao, Z.; Liu, X.; Liu, X. Methyl Jasmonate Induces Apoptosis and Pro-Apoptotic Autophagy via the ROS Pathway in Human Non-Small Cell Lung Cancer. Am. J. Cancer Res. 2016, 6, 187–199.
- Škubník, J.; Pavlíčková, V.; Ruml, T.; Rimpelová, S. Current Perspectives on Taxanes: Focus on Their Bioactivity, Delivery and Combination Therapy. Plants 2021, 10, 569.
- Heyfets, A.; Flescher, E. Cooperative Cytotoxicity of Methyl Jasmonate with Anti-Cancer Drugs and 2-Deoxy-D-Glucose. Cancer Lett. 2007, 250, 300–310.
- Yeruva, L.; Hall, C.; Elegbede, J.A.; Carper, S.W. Perillyl Alcohol and Methyl Jasmonate Sensitize Cancer Cells to Cisplatin. Anticancer. Drugs 2010, 21, 1–9.
- Elia, U.; Flescher, E. PI3K/Akt Pathway Activation Attenuates the Cytotoxic Effect of Methyl Jasmonate toward Sarcoma Cells. Neoplasia 2008, 10, 1303–1313.
- Raviv, Z.; Zilberberg, A.; Cohen, S.; Reischer-Pelech, D.; Horrix, C.; Berger, M.; Rosin-Arbesfeld, R.; Flescher, E. Methyl Jasmonate Down-Regulates Survivin Expression and Sensitizes Colon Carcinoma Cells towards TRAIL-Induced Cytotoxicity. Br. J. Pharmacol. 2011, 164, 1433–1444.
- Jiang, G.; Zhao, J.; Xiao, X.; Tao, D.; Gu, C.; Tong, Q.; Luo, B.; Wang, L.; Zeng, F. AN N-Terminal Smac Peptide Sensitizes Human Prostate Carcinoma Cells to Methyl Jasmonate-Induced Apoptosis. Cancer Lett. 2011, 302, 37–46.
- Yousefi, S.; Darvishi, P.; Yousefi, Z.; Pourfathollah, A.A. Effect of Methyl Jasmonate and 3-Bromopyruvate Combination Therapy on Mice Bearing the 4 T1 Breast Cancer Cell Line. J. Bioenerg. Biomembr. 2020, 52, 103–111.
- Milrot, E.; Jackman, A.; Flescher, E.; Gonen, P.; Kelson, I.; Keisari, Y.; Sherman, L. Enhanced Killing of Cervical Cancer Cells by Combinations of Methyl Jasmonate with Cisplatin, X or Alpha Radiation. Invest. New Drugs 2013, 31, 333–344.
- Guimarães, P.P.G.; Gaglione, S.; Sewastianik, T.; Carrasco, R.D.; Langer, R.; Mitchell, M.J. Nanoparticles for Immune Cytokine TRAIL-Based Cancer Therapy. ACS Nano 2018, 12, 912–931.
- Twomey, J.D.; Kim, S.-R.; Zhao, L.; Bozza, W.P.; Zhang, B. Spatial Dynamics of TRAIL Death Receptors in Cancer Cells. Drug Resist. Updat. Rev. Comment. Antimicrob. Anticancer Chemother. 2015, 19, 13–21.
- Xiao, X.; Jiang, G.; Wang, L.; Lv, L.; Zeng, F.-Q. Predominant Enhancement of Apoptosis Induced by Methyl Jasmonate in Bladder Cancer Cells: Therapeutic Effect of the Antp-Conjugated Smac Peptide. Anticancer. Drugs 2011, 22, 853–863.
