Immunotherapy has changed the environment of cancer treatment by providing new and efficacious therapy options for many solid and hematologic malignancies. Although not a new field of oncology, immunotherapy has quickly developed into one of the most flourishing fields in medicine. In this review article, we explore key discoveries which helped to shape our current understanding of the immune system’s role in neoplasms. Many landmark developments include the advancements in checkpoint inhibitors, monoclonal antibodies, CAR-T cells and anti-cancer vaccines. We also explore the drawbacks and efficacy of various categories of immunotherapy. Ongoing investigations within immunotherapy, such as the gut microbiome, combining checkpoint inhibitors and gene sequencing, continue to personalize treatments for cancer patients, providing exciting and endless possibilities for the future.
- vaccines
- immunotherapy
- cancer
1. Introduction
2. Cytokine Therapy
3. Checkpoint Inhibition
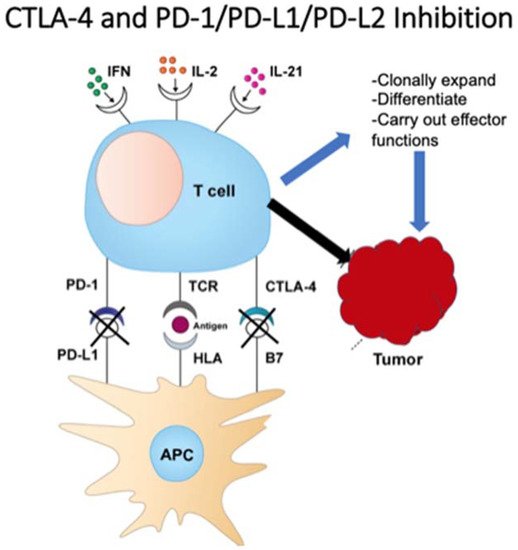
4. Antitumor Monoclonal Antibodies
| Drug Name | Target | Malignancy Approved (Single Agent or in Combination) |
Approval Year |
|---|---|---|---|
| Atezolizumab | PD-L1 | Multiple solid tumors | 2016 |
| Bevacizumab | VEGF-A | Multiple solid tumors | 2004 |
| Brentuximab vedotin | CD30 | Hodgkin’s lymphoma; Anaplastic LCL; PTCL | 2011 |
| Blinatumomab | CD3, CD19 | Acute lymphoblastic leukemia | 2014 |
| Cemiplimab | PD-1 | Cutaneous squamous cell carcinoma | 2018 |
| Cetuximab | EGFR | Colorectal cancer (K-RAS wildtype); NSCLC, SCC head and neck | 2004 |
| Daratumumab | CD38 | Multiple myeloma | 2015 |
| Durvalumab | PD-L1 | Urothelial carcinoma, NSCLC, small cell lung cancer | 2017 |
| Elotuzumab | SLAMF7 | Multiple myeloma | 2015 |
| Gemtuzumab ozogamicin | CD33 | Acute myeloid leukemia | 2000 |
| Ipilimumab | CTLA-4 | Multiple solid tumors | 2011 |
| Isatuximab | CD38 | Multiple myeloma | 2020 |
| Mogamilizumab | CCR4 | Mycosis fungoides or Sezary syndrome, CTCL, T cell leukemia/lymphoma | 2018 |
| Nivolumab | PD-1 | Multiple solid tumors | 2014 |
| Obinutuzumab | CD20 | Chronic lymphocytic leukemia, follicular lymphoma | 2013 |
| Panitumumab | EGFR | Colorectal cancer | 2006 |
| Pembrolizumab | PD-1 | Multiple solid tumors | 2014 |
| Pertuzumab | HER2 | Breast cancer (HER2+) | 2012 |
| Ramucirumab | VEGFR2 | Multiple solid tumors | 2014 |
| Rituximab | CD20 | Multiple hematologic malignancies and autoimmune diseases | 1997 |
| Trastuzumab | HER2 | Breast cancer (HER2+), gastric/GEJ adenocarcinoma (HER2+) | 1998 |
References
- Ichim, C.V. Revisiting immunosurveillance and immunostimulation: Implications for cancer immunotherapy. J. Transl. Med. 2005, 3, 8.
- Coley, W.B. Contribution to the Knowledge of Sarcoma. Ann. Surg. 1891, 14, 199–220.
- McCarthy, E.F. The toxins of William B. Coley and the treatment of bone and soft-tissue sarcomas. Iowa Orthop. J. 2006, 26, 154–158.
- Burnet, M. Cancer—A Biological Approach: III. Viruses Associated with Neoplastic Conditions. IV. Practical Applications. BMJ 1957, 1, 841–847.
- Thomas, L.; Lawrence, H.S. Cellular and Humoral Aspects of the Hypersensitive States; Hoeber-Harper: New York, NY, USA, 1959.
- Teng, M.W.L.; Kershaw, M.H.; Smyth, M.J. Chapter 7—Cancer Immunoediting: From Surveillance to Escape. In Cancer Immunotherapy, 2nd ed.; Prendergast, G.C., Jaffee, E.M., Eds.; Academic Press: New York, NY, USA, 2013; pp. 85–99. ISBN 9780123942968.
- Houghton, A.N.; Guevara-Patiño, J.A. Immune recognition of self in immunity against cancer. J. Clin. Investig. 2004, 114, 468–471.
- Kim, R.; Emi, M.; Tanabe, K. Cancer immunoediting from immune surveillance to immune escape. Immunology 2007, 121, 1–14.
- Jiang, T.; Zhou, C.; Ren, S. Role of IL-2 in cancer immunotherapy. OncoImmunology 2016, 5, e1163462.
- Osipov, A.; Murphy, A.; Zheng, L. From immune checkpoints to vaccines: The past, present and future of cancer immunotherapy. Adv. Cancer Res. 2019, 143, 63–144.
- Morgan, D.A.; Ruscetti, F.W.; Gallo, R. Selective in vitro growth of T lymphocytes from normal human bone marrows. Science 1976, 193, 1007–1008.
- Abbas, A.K.; Trotta, E.; Simeonov, D.R.; Marson, A.; Bluestone, J.A. Revisiting IL-2: Biology and therapeutic prospects. Sci. Immunol. 2018, 3, eaat1482.
- Taniguchi, T.; Matsui, H.; Fujita, T.; Takaoka, C.; Kashima, N.; Yoshimoto, R.; Hamuro, J. Structure and expression of a cloned cDNA for human interleukin-2. Nat. Cell Biol. 1983, 302, 305–310.
- Rosenberg, S.A.; Mulé, J.J.; Spiess, P.J.; Reichert, C.M.; Schwarz, S.L. Regression of established pulmonary metastases and subcutaneous tumor mediated by the systemic administration of high-dose recombinant interleukin 2. J. Exp. Med. 1985, 161, 1169–1188.
- Klapper, J.A.; Downey, S.G.; Smith, F.O.; Yang, J.C.; Hughes, M.S.; Kammula, U.S.; Sherry, R.M.; Royal, R.E.; Steinberg, S.M.; Rosenberg, S. High-dose interleukin-2 for the treatment of metastatic renal cell carcinoma. Cancer 2008, 113, 293–301.
- Dutcher, J.P.; Schwartzentruber, D.J.; Kaufman, H.L.; Agarwala, S.S.; Tarhini, A.A.; Lowder, J.N.; Atkins, M.B. High dose interleukin-2 (Aldesleukin)-expert consensus on best management practices-2014. J. Immunother. Cancer 2014, 2, 26.
- Pardoll, D.M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 2012, 12, 252–264.
- Buchbinder, E.I.; Desai, A. CTLA-4 and PD-1 Pathways. Am. J. Clin. Oncol. 2016, 39, 98–106.
- Chambers, C.A.; Kuhns, M.S. CTLA-4-Mediated Inhibition in Regulation of T Cell Responses: Mechanisms and Manipulation in Tumor Immunotherapy. Annu. Rev. Immunol. 2001, 19, 565–594.
- Leach, D.R.; Krummel, M.F.; Allison, J.P. Enhancement of Antitumor Immunity by CTLA-4 Blockade. Science 1996, 271, 1734–1736.
- U.S. Food and Drug Administration. FDA Approves New Treatment for a Type of Late-Stage Skin Cancer. Available online: (accessed on 5 March 2021).
- Tarhini, A.; Lo, E.; Minor, D.R. Releasing the Brake on the Immune System: Ipilimumab in Melanoma and Other Tumors. Cancer Biother. Radiopharm. 2010, 25, 601–613.
- Ishida, Y.; Agata, Y.; Shibahara, K.; Honjo, T. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J. 1992, 11, 3887–3895.
- George, A.P.; Kuzel, T.M.; Zhang, Y.; Zhang, B. The Discovery of Biomarkers in Cancer Immunotherapy. Comput. Struct. Biotechnol. J. 2019, 17, 484–497.
- Keegan, P. Center for Drug Evaluation and Research Summary Review-125514Orig1s000. September 2014. Available online: (accessed on 11 May 2021).
- Tan, S.; Li, D.; Zhu, X. Cancer immunotherapy: Pros, cons and beyond. Biomed. Pharmacother. 2020, 124, 109821.
- Puri, S.; Shafique, M. Combination checkpoint inhibitors for treatment of non-small-cell lung cancer: An update on dual anti-CTLA-4 and anti-PD-1/PD-L1 therapies. Drugs Context 2020, 9.
- PD-1/PD-L1 Landscape. Cancer Research Institute. Available online: (accessed on 5 March 2021).
- Zhao, J.; Chen, Y.; Ding, Z.-Y.; Liu, J.-Y. Safety and Efficacy of Therapeutic Cancer Vaccines Alone or in Combination with Immune Checkpoint Inhibitors in Cancer Treatment. Front. Pharmacol. 2019, 10.
- Köhler, G.; Milstein, C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nat. Cell Biol. 1975, 256, 495–497.
- Pierpont, T.M.; Limper, C.B.; Richards, K.L. Past, Present, and Future of Rituximab—The World’s First Oncology Monoclonal Antibody Therapy. Front. Oncol. 2018, 8, 163.
- Cruz, E.; Kayser, V. Monoclonal antibody therapy of solid tumors: Clinical limitations and novel strategies to enhance treatment efficacy. Biol. Targets Ther. 2019, 13, 33–51.
- Oostra, D.R.; Macrae, E.R. Role of trastuzumab emtansine in the treatment of HER2-positive breast cancer. Breast Cancer Targets Ther. 2014, 6, 103–113.
