Human platelet lysate (HPL), a biological material derived from platelet concentrates, can be manufactured as a cost-effective and standardized cell culture supplement
[5][6][5,6]. HPL is known to contain abundant mitogenic growth factors, including vascular endothelial growth factor (VEGF), basic fibroblast growth factor (bFGF), epidermal growth factor (EGF), platelet-derived growth factors (PDGF) and transforming growth factor (TGF)
[7][8][7,8]. These growth factors not only exert a positive effect on angiogenesis and post-ischemic vascular remodeling but also play a critical role in tissue inflammation and regeneration
[9][10][11][9,10,11]. Therefore, platelet lysates have demonstrated the advantages of replacing fetal bovine serum (FBS) for culturing various types of cells, including stem cells, fibroblasts and endothelial cells
[1][12][1,12]. The use of HPL in cell culture is desired for clinical use by circumventing the risks of xeno-immunization against bovine antigens and the transmission of zoonotic diseases
[13][14][13,14]. Moreover, the effect of HPL on directly promoting angiogenesis and tissue regeneration has been demonstrated
[15][16][15,16], so HPL holds great promise for treating various tissue defects.
The development of efficient biomaterials to assist HPL delivery for tissue regeneration remains a challenge. To facilitate a sustained release of its contents in the defect site, HPL could be blended with biomaterials, adsorbed onto scaffolds or used as a gel to mediate neo-angiogenesis
[17][18][19][17,18,19]. However, the currently available options are not ideal, and a novel hydrogel system is desired to allow sustained HPL delivery. Hydrogel is a
web of crosslinked polymer
filmaments soaked by an aqueous solutionwith a large amount of water but is not soluble in water. It is usually soft and flexible with good fluid-retaining capability and biocompatibility, so it has been widely applied in various biomedical fields as a kind of drug delivery system
[20][21][20,21]. Chitosan-based thermosensitive hydrogels, fabricated with a chitosan/β-glycerophosphate (β-GP) system, have been successfully applied in drug release studies
[22], and they were further applied to deliver cells for tissue engineering purposes
[23][24][23,24]. β-GP acts as a proton receiver from the positively charged chitosan at elevated temperature, thereby inducing chitosan gelation
[25]. Moreover, our previous study demonstrated superior biocompatibility when gelatin was blended into this chitosan/β-GP system
[26].
When gelatin and chitosan are mixed for hydrogel fabrication, they form poly-electrolytic complexes in different gelated states, and the structure mimics the natural components of the extracellular matrix
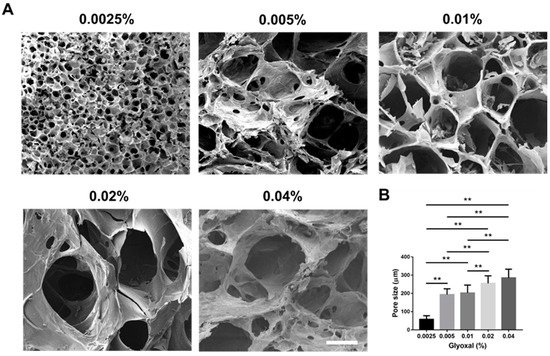
[27][28][27,28]. However, the mechanical property of the composite hydrogel is still not ideal. To achieve better mechanical strength and a controlled release pattern, glyoxal was selected as a crosslinker to reinforce the hydrogel in this study
[29][30][29,30]. Glyoxal is the smallest dialdehyde with high water solubility, and it exhibits lower cytotoxicity when compared to most chemical crosslinkers
[29][30][31][29,30,31]. It was expected to crosslink the available amine groups on chitosan and gelatin chains to form the network
[32], rendering improved mechanical properties of the chitosan/gelatin (CS-GE) hydrogel. In this study, we aimed to fabricate a CS-GE hydrogel crosslinked by glyoxal for the sustained release of HPL. An ideal concentration of glyoxal was selected, resulting in negligible cytotoxicity with a reasonable level of mechanical strength. These characteristics can facilitate efficient HPL delivery from the crosslinked CS-GE hydrogel to promote angiogenesis and regeneration in damaged tissues.
3. Current Insightson
HPL contains lots of well-known platelet growth factors, which have been considered to play an essential role in cell-mediated angiogenesis and skin fibroblast behavior
[33][34][33,34]. Previous publications have shown that HPL can be utilized for the restoration of corneal lesions
[35], diabetic ulcers
[36] and other soft tissue pathologies
[37]. Apart from the soft tissue, HPL can be successfully applied for the local treatment of osteoarthritis, which is attributed to the growth factors released from platelet granules after intra-articular activation
[38]. Mesenchymal stem cells can also be combined with HPL for bone regeneration
[39]. However, HPL-based treatment may be limited because of the poor retention of HPL in the defect sites. In the present study, HPL-incorporated CS-GE hydrogel was successfully fabricated, and its mechanical property was improved by crosslinking with glyoxal. The HPL-incorporated CS-GE hydrogel not only significantly promoted the proliferative activity of Hs68 fibroblasts and the migration of HUVECs in vitro but also enhanced angiogenesis in the in ovo CAM model. Hence, the novel crosslinked hydrogel system allows sustained release of HPL-derived growth factors to promote tissue regeneration.
Chitosan-based hydrogels are often combined with β-GP to constitute a thermally responsive hydrogel system. Due to the electrostatic attractions between the chitosan protonated amine groups (-NH
3+) and negatively charged phosphate molecules of β-GP (-HPO
4− or -PO
42−), the hydrogel system composed of chitosan, gelatin and β-GP can stay in a liquid state without aggregation at low temperature
[40][41][40,41]. By adding β-GP as a weak base, the pH of the solution is escalated close to physiological pH so that the available -NH
2 on the chitosan and gelatin chains may increase
[42]. Therefore, the more available reaction sites can be bound by adding glyoxal as a crosslinker to form a robust network structure. Consequently, a higher glyoxal concentration led to greater mechanical strength and a shorter gelation time (less than ~70 s) in this study.
Recently, glyoxal has been used as an alternative dialdehyde crosslinker in various biomedical applications
[42][43][42,43]. Although a higher amount of glyoxal was reported to trigger oxidative stress, thereby inducing cytotoxicity
[44], we demonstrated no significant cytotoxicity if the concentration of glyoxal was less than 0.01% in cell culture. Compared to other chemical crosslinkers for CS-GE hydrogels, glyoxal exhibits several advantages for its application in tissue engineering and drug delivery. Genipin-crosslinked chitosan-based hydrogels were shown to have good biocompatibility and low cytotoxicity, but the gelation time was at least 1 h, rendering it not suitable for clinical use
[45][46][45,46]. Moreover, an anti-angiogenesis effect of genipin was reported to decrease the rate of wound healing
[47]. Glutaraldehyde can crosslink chitosan chains to form hydrogels within 1 h; however, it is considered toxic for the respiratory tract, eyes and skin
[48].
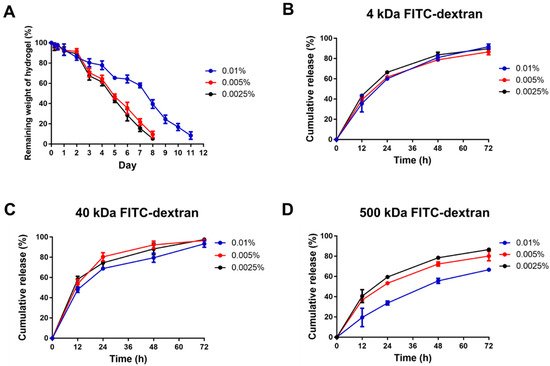
Our previous study manifested blending gelatin in the chitosan-based hydrogel, resulting in a more porous architecture after in vivo application due to the degradation of gelatin
[26]. This porous structure can ensure that more growth factors are released rather than stuck in the hydrogel. By modifying this design in the present study, the chemical crosslinker, glyoxal, was used to increase the mechanical strength of the hydrogel, rendering a more sustained release of the encapsulated HPL. Lim et al. fabricated HPL-incorporated hydroxybutyl chitosan hydrogels, which displayed a cumulative release of more than 80% of PDGF-BB and TGF-β1 on day 7 during in vitro degradation
[49]. Compared to their result, the cumulative releases of PDGF-BB and TGF-β1 in our experiment were less than 80%, demonstrating a more sustained release of HPL.
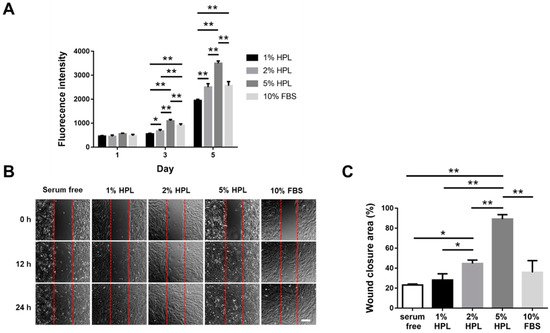
In this study, a significantly higher cell proliferative activity was noted as the HPL concentration increased in the medium. In line with the previous publications suggesting that HPL is more suitable than FBS as culture supplements
[50][51][50,51], our results revealed that 5% HPL was significantly more effective than 10% FBS to promote Hs68 fibroblast proliferation and migration. Li et al. have elucidated the mechanism of human dermal fibroblast migration driven by PDGF-BB, indicating the important role of PDGF in regulating the fibroblast behavior
[52]. HPL exhibits abundant PDGF-BB, indicating the great potential of HPL in fibroblast-driven soft tissue regeneration, such as wound healing.
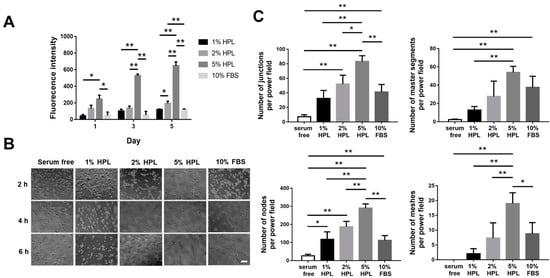
Tissue regeneration normally needs the formation of vessels to transfer essential materials including oxygen and nutrition. Therefore, capillary angiogenesis within the damaged tissue is critical during the tissue repair process. Angiogenesis is a coordinated process, involving endothelial cellular proliferation, migration and association in tubular structures
[53]. In this work, HUVECs cultured in the medium containing 5% HPL revealed significantly more tube-like structures relative to the 10% FBS group, and the HPL-incorporated hydrogel significantly induced the migration of HUVECs. Moreover, the CAM assay was employed for further evaluation because it can be applied to study in ovo both angiogenesis and anti-angiogenesis in response to different materials
[54].