Sorsby‘s fundus dystrophy (SFD) is a rare, autosomal dominant inherited retinal disease with complete penetrance affecting both genders similarly, typically becoming symptomatic after the second decade of life, with an average onset in the 4th to 5th decade of life, leading to severe bilateral vision loss and blindness if left untreated.
- Sorsby’s fundus dystrophy
- Sorsby
- hereditary retinal dystrophy
- choroidal neovascularisation
- macular neovascularization
- anti-VEGF treatment
- long-term FU
- treatment outcome
1. Introduction
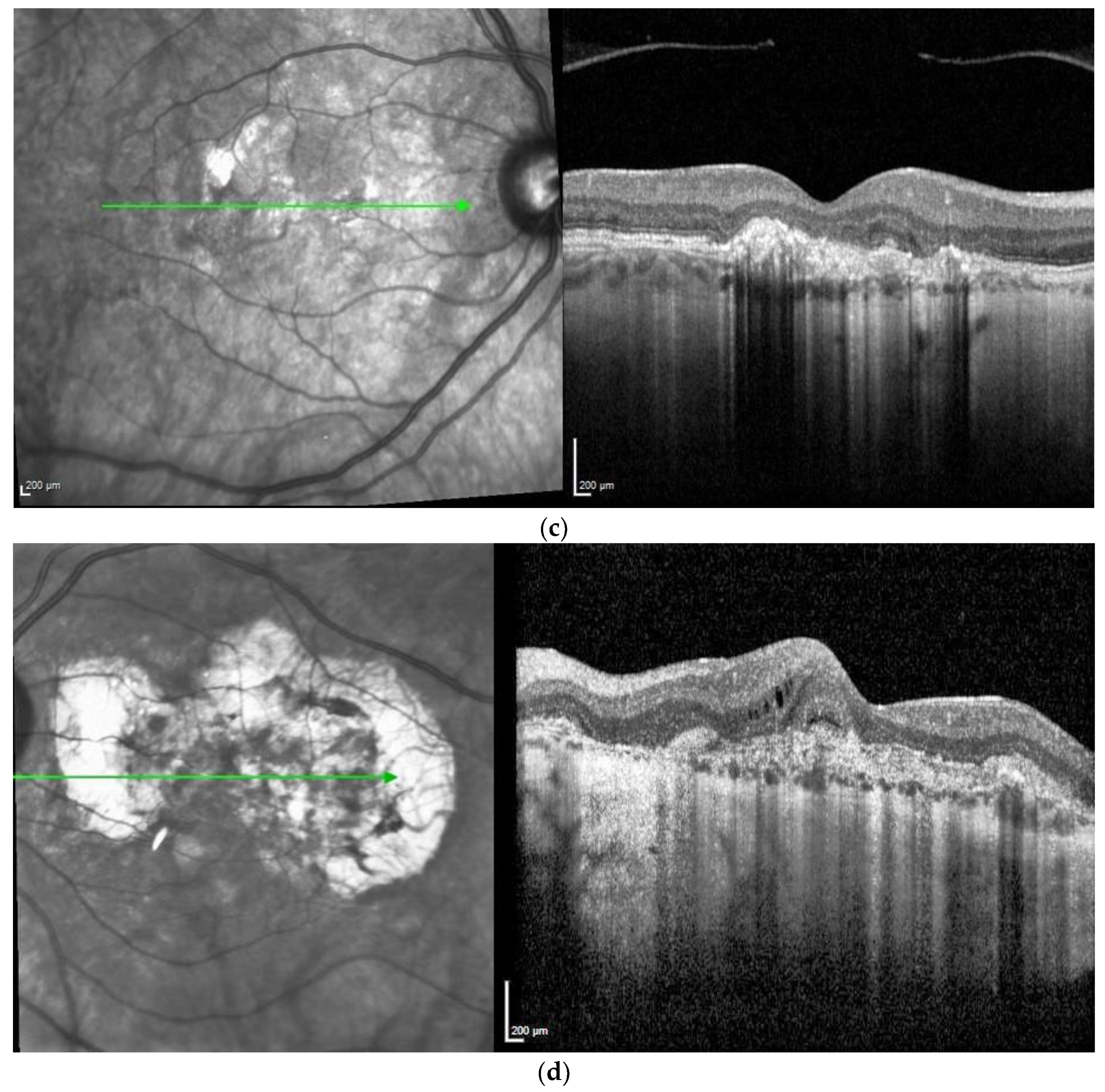
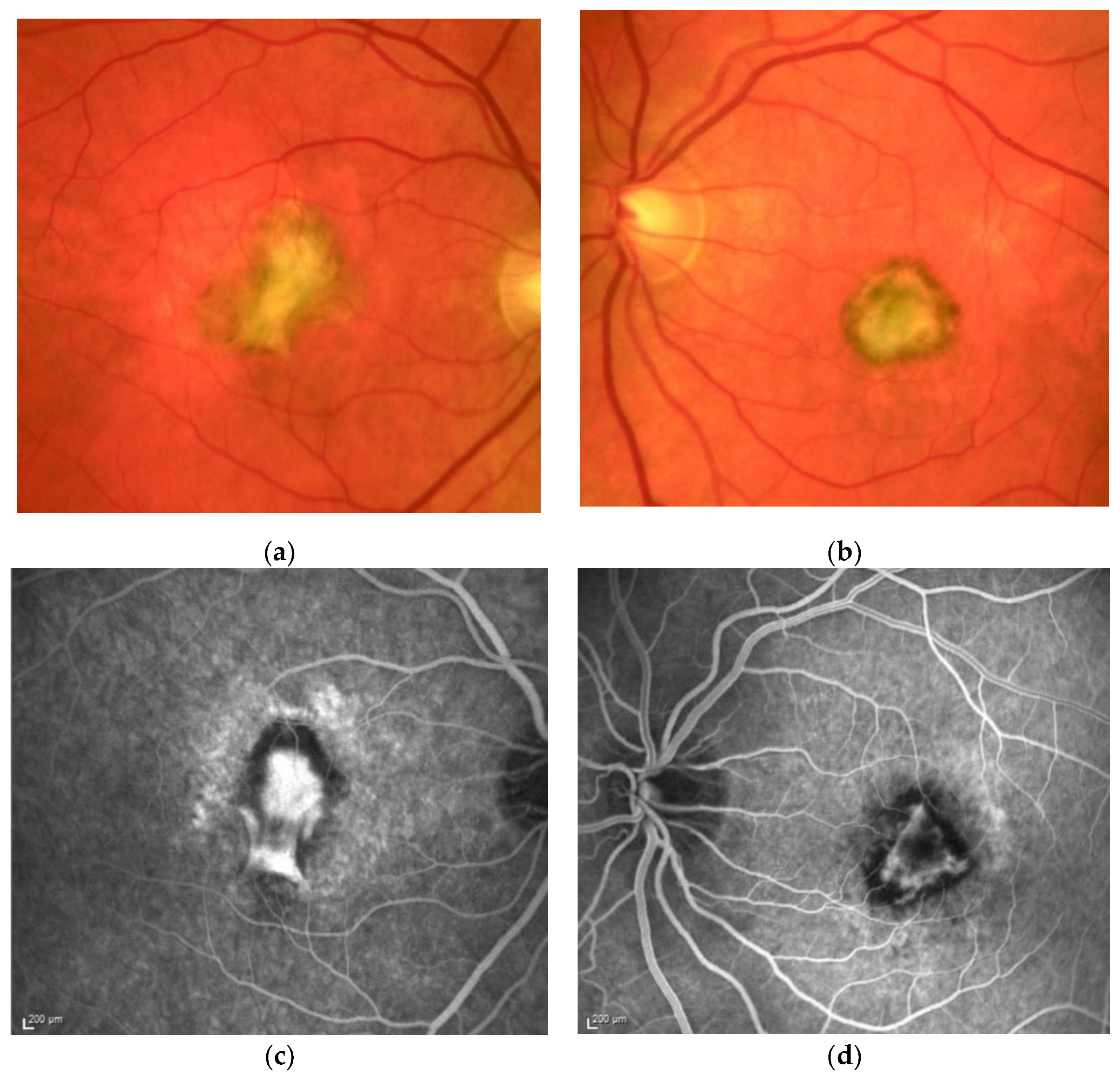 Figure 1. M, 35 years, M. Sorsby. Clinical image of both eyes with a significant submacular fibrovascular lesion after three courses of photodynamic therapy in the right (a) and four in the left eye (b), prior to the start of intravitreal therapy. (second panel). Same patient, fluorescein angiography (R middle (c), L early arteriovenous phase (d)) confirming a low-active predominantly classic macular neovascularisation.
Figure 1. M, 35 years, M. Sorsby. Clinical image of both eyes with a significant submacular fibrovascular lesion after three courses of photodynamic therapy in the right (a) and four in the left eye (b), prior to the start of intravitreal therapy. (second panel). Same patient, fluorescein angiography (R middle (c), L early arteriovenous phase (d)) confirming a low-active predominantly classic macular neovascularisation.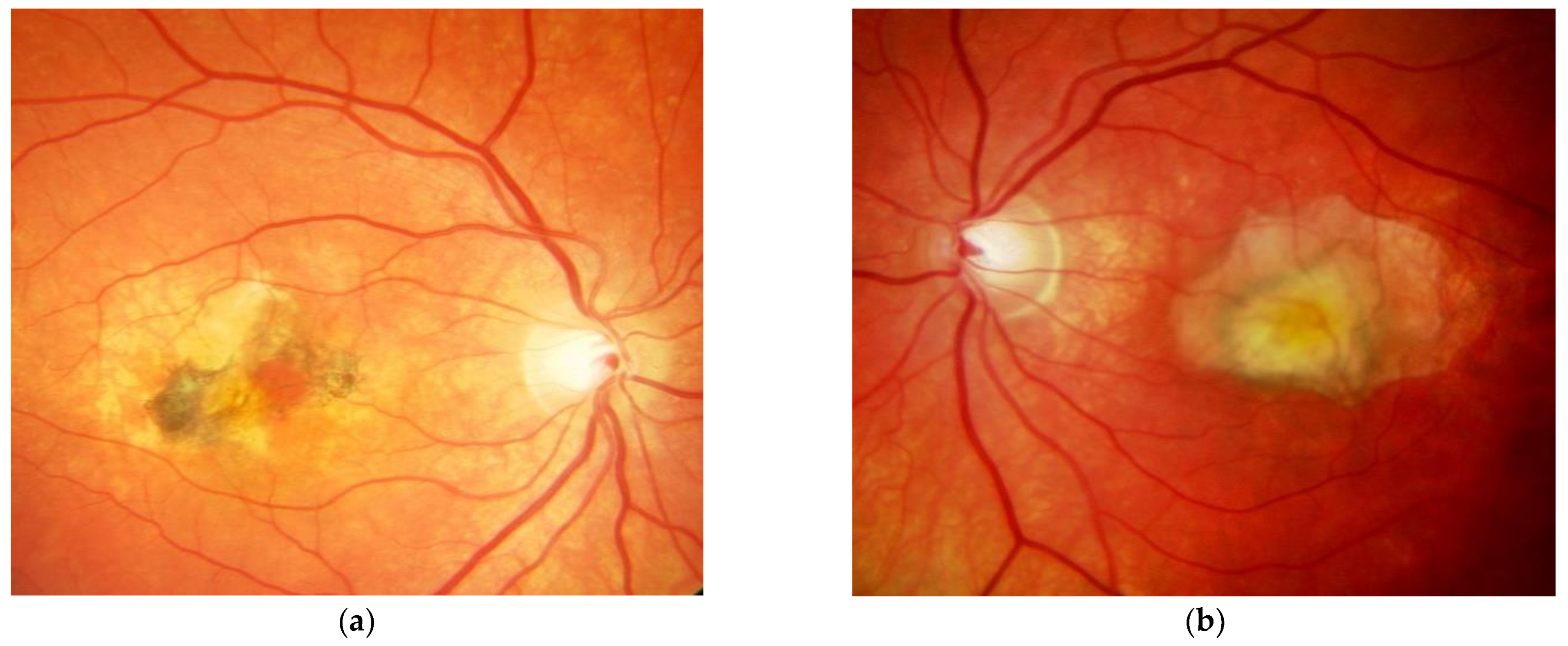 Figure 2. Same patient, 5 years later. First reactivation of macular neovascularisation evidenced by vision loss and a small macular hemorrhage as well as newly present intraretinal fluid in OCT in the right eye (a) and macular pigment atrophy in both eyes (a,b).
Figure 2. Same patient, 5 years later. First reactivation of macular neovascularisation evidenced by vision loss and a small macular hemorrhage as well as newly present intraretinal fluid in OCT in the right eye (a) and macular pigment atrophy in both eyes (a,b).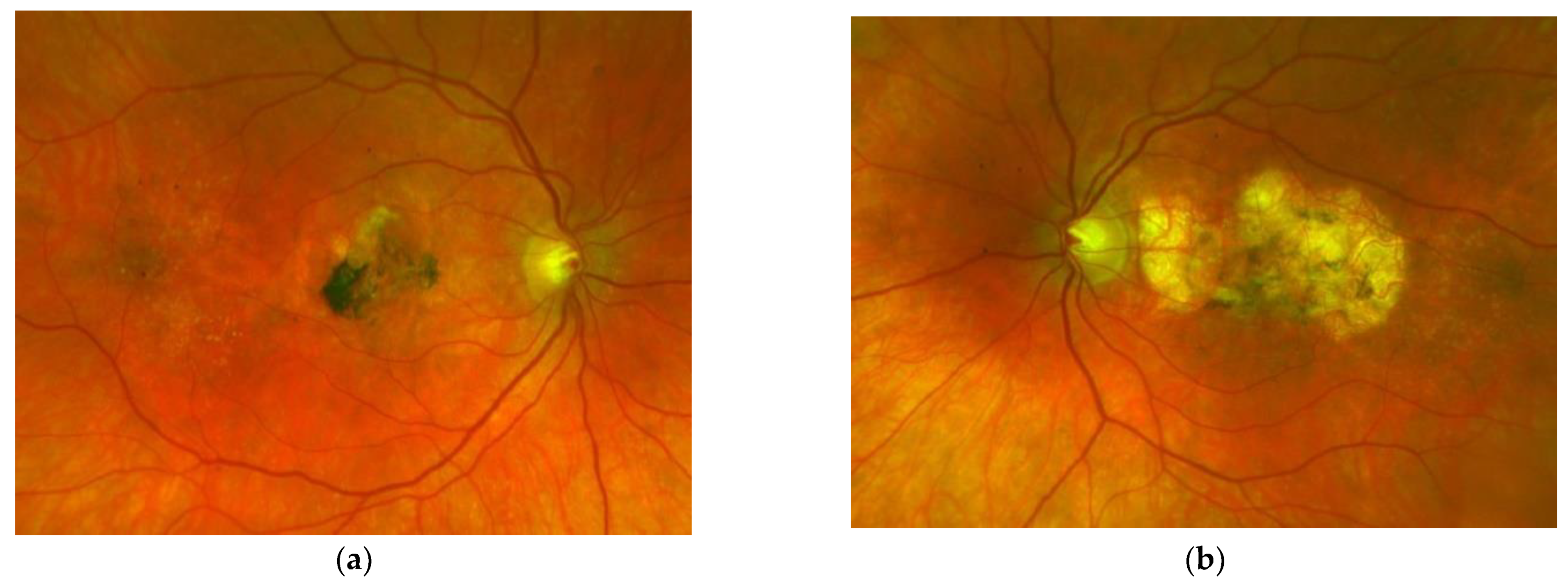 Figure 3. Same patient, 2016, 10 years after the start of intravitreal therapy; no lesion activity after 22 intravitreal Ranibizumab injections in the right eye (a) and a remarkable progressive macular atrophy despite a stable scar in his left eye (b).
Figure 3. Same patient, 2016, 10 years after the start of intravitreal therapy; no lesion activity after 22 intravitreal Ranibizumab injections in the right eye (a) and a remarkable progressive macular atrophy despite a stable scar in his left eye (b).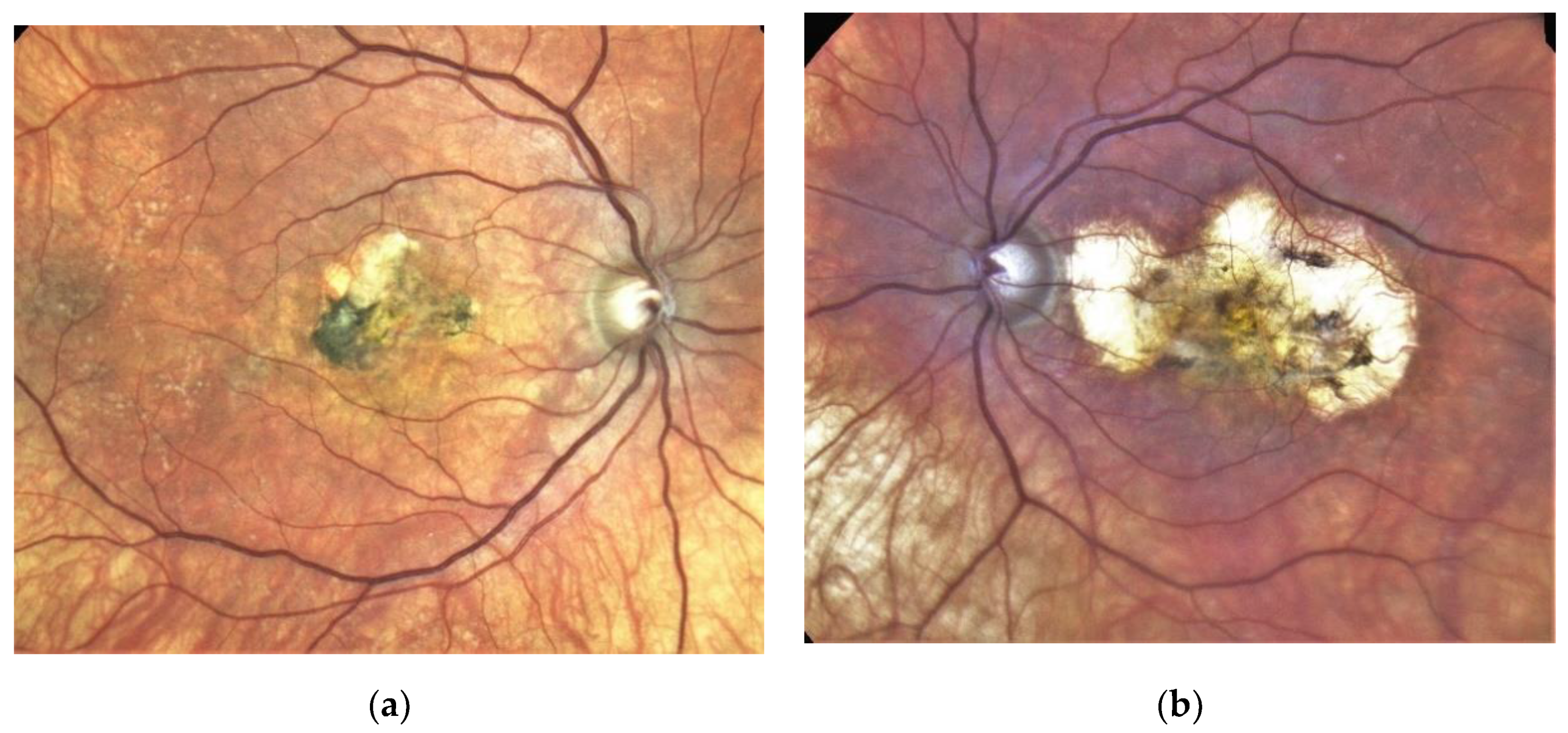
2. Case report
A 51-year-old male presented at the age of 33 years with a first episode of simultaneous vision loss in both eyes in January 2004. He related a positive family history of SFD, as his father, uncle, as well as other first-degree relatives, had previously been diagnosed with SFD. Fluorescein angiography demonstrated a juxtafoveal, classical MNV in the right eye and a small, subfoveal, predominantly classic MNV in the left eye. Genetic testing revealed a mutation in the TIMP3 gene. Until January 2005, the patient received three and four sessions of full-fluence PDT using a standard dose (6mg) of verteporfin (Novartis Inc., Basel, Switzerland) in his right and left eye each, respectively, along with parabulbar triamcinolone injections. With treatment, a stable course in the right eye contrasted to repeated re-activations of the subretinal MNV in his left eye. Therefore, he received four intravitreal injections of crystalline triamcinolone (8mg) in his left eye in the following 15 months, accompanied by a rise of intraocular pressure and cataract formation. By that time, central vision in his left eye had dropped to Snellen 0.20 due to progressive macular scarring and pigment epitheliopathy in both eyes. After the introduction of intravitreal anti-VEGF therapy for exudative maculopathy, the patient received a total of nine injections of Ranibizumab (Ran; Lucentis®, Genentech, South San Francisco, CA, USA) to control disease activity in his left eye between 2007–2012. After lesion inactivity due to macular scarring had been observed over one year, intravitreal therapy was ceased at a central Snellen vision of 0.16.
In April 2011, the patient presented with a symptomatic MNV reactivation of his right eye and 0.5 mg Ranibizumab intravitreally was initiated and continued following a pro re nata (PRN) scheme. Since April 2011, a total of 24 Ranibizumab injections were administered to the right eye, with 22 of these administered up until July 2015. The last injection was administered in July 2019. During the last FU in February 2021, the patient had a best corrected Snellen VA of 1.0 in his right eye and 0.16 in his left eye. Though functional stability was achieved over the last 10 years, progression of severity and extension of RPE changes, Drusen formation and choroidal sclerosis was evident in both eyes (Figures 1, 2, 3 and 4). Nevertheless, with consequent clinical controls and Ranibizumab treatment immediately upon first signs of lesion reactivation, his quality of life is perceived as excellent, he can follow his daily professional and private activities without relevant restrictions.
3. Visual Outcome after Intravitreal Anti-VEGF Therapy for Macular Neovascularisation Secondary to Sorsby’s Fundus Dystrophy
References
- Jacobson, S.G.; Cideciyan, A.V.; Regunath, G.; Rodriguez, F.J.; VanDenburgh, K.; Sheffield, V.C.; Stone, E.M. Night blindness in Sorsby’s fundus dystrophy reversed by vitamin A. Nat. Genet. 1995, 11, 27–32.
- Handsley, M.M.; Edwards, D.R. Metalloproteinases and their inhibitors in tumor angiogenesis. Int. J. Cancer 2005, 115, 849–860.
- Qi, J.H.; Ebrahem, Q.; Ali, M.; Cutler, A.; Bell, B.; Prayson, N.; Sears, J.; Knauper, V.; Murphy, G.; Anand-Apte, B. Tissue Inhibitor of Metalloproteinases-3 Peptides Inhibit Angiogenesis and Choroidal Neovascularization in Mice. PLoS ONE 2013, 8, e55667.
- Qi, J.H.; Ebrahem, Q.; Moore, N.; Murphy, G.; Claesson-Welsh, L.; Bond, M.; Baker, A.; Anand-Apte, B. A novel function for tissue inhibitor of metalloproteinases-3 (TIMP3): Inhibition of angiogenesis by blockage of VEGF binding to VEGF receptor-2. Nat. Med. 2003, 9, 407–415.
- Fariss, R.N.; Apte, S.S.; Olsen, B.R.; Iwata, K.; Milam, A.H. Tissue inhibitor of metalloproteinases-3 is a compo-nent of Bruch’s membrane of the eye. Am. J. Pathol. 1997, 150, 323–328.
- Langton, K.P.; McKie, N.; Smith, B.M.; Brown, N.J.; Barker, M.D. Sorsby’s fundus dystrophy mutations impair turnover of TIMP-3 by retinal pigment epithelial cells. Hum. Mol. Genet. 2005, 14, 3579–3586.
- Christensen, D.R.; Brown, F.E.; Cree, A.J.; Ratnayaka, J.A.; Lotery, A.J. Sorsby fundus dystrophy—A review of pathology and disease mechanisms. Exp. Eye Res. 2017, 165, 35–46.
- Anand-Apte, B.; Chao, J.R.; Singh, R.; Stöhr, H. Sorsby fundus dystrophy:Insights from the past and looking to the future. J. Neurosci. Res. 2018, 97, 88–97.
- Sivaprasad, S.; Webster, A.R.; Egan, C.; Bird, A.C.; Tufail, A. Clinical Course and Treatment Outcomes of Sorsby Fundus Dystrophy. Am. J. Ophthalmol. 2008, 146, 228–234.e2.
- Daniel, E.; Toth, C.A.; Grunwald, J.E.; Jaffe, G.J.; Martin, D.F.; Fine, S.L.; Huang, J.; Ying, G.-S.; Hagstrom, S.A.; Winter, K.; et al. Risk of Scar in the Comparison of Age-related Macular Degeneration Treatments Trials. Ophthalmology 2014, 121, 656–666.
- Capon, M.R.C.; Polkinghorne, P.J.; Fitzke, F.W.; Bird, A.C. Sorsby’s pseudoinflammatory macula dystrophy—Sorsby’s fundus dystrophies. Eye 1988, 2, 114–122.
- Hamilton, W.K.; Ewing, C.C.; Ives, E.J.; Carruthers, J.D. Sorsby’s Fundus Dystrophy. Ophthalmology 1989, 96, 1755–1762.
- Polkinghorne, P.J.; Capon, M.R.; Berninger, T.; Lyness, A.L.; Sehmi, K.; Bird, A.C. Sorsby’s Fundus Dystrophy: A clinical study. Ophthalmology 1989, 96, 1763–1768.
- Gliem, M.; Müller, P.L.; Mangold, E.; Bolz, H.J.; Stöhr, H.; Weber, B.H.; Holz, F.G.; Issa, P.C. Reticular Pseudodrusen in Sorsby Fundus Dystrophy. Ophthalmology 2015, 122, 1555–1562.
- Menassa, N.; Burgula, S.; Empeslidis, T.; Tsaousis, K.T. Bilateral choroidal neovascular membrane in a young patient with Sorsby fundus dystrophy: The value of prompt treatment. BMJ Case Rep. 2017, 2017, 2017220488.
- Gliem, M.; Müller, P.L.; Mangold, E.; Holz, F.G.; Bolz, H.J.; Stöhr, H.; Weber, B.H.F.; Issa, P.C. Sorsby Fundus Dystrophy: Novel Mutations, Novel Phenotypic Characteristics, and Treatment Outcomes. Investig. Opthalmol. Vis. Sci. 2015, 56, 2664–2676.
- Holz, F.G.; Haimovici, R.; Wagner, D.G.; Bird, A.C. Recurrent Choroidal Neovascularization after Laser Photocoagulation in Sorsbyʼs Fundus Dystrophy. Retina 1994, 14, 329–334.
- Keller, J.; Giralt, J.; Alforja, S.; Casaroli-Marano, R.P. Altering the Clinical Course of Sorsby Fundus Dystrophy with the Use of Anti-Vascular Endothelial Growth Factor Intraocular Therapy. Retin. Cases Brief Rep. 2015, 9, 104–105.
- Spaide, R.F. Long-Term Visual Acuity Preservation in Sorsby Fundus Dystrophy with Corticosteroid Treatment. Retin. Cases Brief Rep. 2019.
- Fung, A.T.; Stöhr, H.; Weber, B.H.F.; Holz, F.G.; Yannuzzi, L.A. Atypical Sorsby Fundus Dystrophy with a Novel Tyr159cys Timp-3 Mutation. Retin. Cases Brief Rep. 2013, 7, 71–74.
- Kapoor, K.G.; Bakri, S.J. Intravitreal Anti-Vascular Endothelial Growth Factor Therapy for Choroidal Neovascularization Due to Sorsby Macular Dystrophy. J. Ocul. Pharmacol. Ther. 2013, 29, 444–447.
- Peiretti, E.; Klancnik, J.M.; Spaide, R.F.; Yannuzzi, L. Choroidal Neovascularization in Sorsby Fundus Dystrophy Treated with Photodynamic Therapy and Intravitreal Triamcinolone Acetonide. Retina 2005, 25, 377–379.
- Wong, S.C.; Fong, K.C.S.; Lee, N.; Gregory-Evans, K.; Gregory-Evans, C.Y. Successful photodynamic therapy for subretinal neovascularisation due to Sorsby’s fundus dystrophy: 1 year follow up. Br. J. Ophthalmol. 2003, 87, 796–797.
- Mohla, A.; Khan, K.; Kasilian, M.; Michaelides, M. OCT angiography in the management of choroidal neovascular membrane secondary to Sorsby fundus dystrophy. BMJ Case Rep. 2016, 2016, 2016216453.
- Tsokolas, G.; Almuhtaseb, H.; Lotery, A. Evaluation of Pro-re-Nata (PRN) and Treat and Extend Bevacizumab treatment protocols in Sorsby Fundus Dystrophy. Eur. J. Ophthalmol. 2018, 30, 26–33.
- Gemenetzi, M.K.; Luff, A.J.; Lotery, A.J. Successful Treatment of Choroidal Neovascularization Secondary to Sorsby Fundus Dystrophy with Intravitreal Bevacizumab. Retin. Cases Brief Rep. 2011, 5, 132–135.
- Gray, T.L.; Wong, H.-C.; Raymond, G.L. Choroidal Neovascularization Secondary to Sorsby Fundus Dystrophy Treated with Intravitreal Bevacizumab. Retin. Cases Brief Rep. 2012, 6, 193–196.
- Balaskas, K.; Hovan, M.; Mahmood, S.; Bishop, P. Ranibizumab for the management of Sorsby fundus dystrophy. Eye 2012, 27, 101–102.
- Copete-Piqueras, S.; Cava-Valenciano, C.; Flores-Moreno, I.; Moreno-Valladares, A.; Ruescas, V.B. Tratamiento antiangiogénico en fondo de distrofia de Sorsby sin mutación en gen de TIMP-3. Arch. Soc. Española Oftalmol. 2013, 88, 240–243.
- Sanz, G.F.; Alonso-Gonzalez, R.; Keane, P.; Carreno, E.; Liew, G.; Sim, D.; Patel, P.; Webster, A.; Egan, C.; Tufail, A. Treatment with Intravitreal An-ti-VEGF for Choroidal Neovascular Membrane secondary to Sorsby’s Fundus Dystrophy: A 24-Month Analysis. Investig. Ophthalmol. Vis. Sci. 2013, 54, 3863.
- Kaye, R.; Lotery, A. Long-term Outcome of Bevacizumab Therapy in Sorsby Fundus Dystrophy, A Case Series. Investig. Ophthalmol. Vis. Sci. 2017, 58, 229.
