Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Andrea Ehrmann and Version 2 by Peter Tang.
Electrospinning can be used to prepare nanofiber mats from diverse polymers, polymer blends, or polymers doped with other materials. Amongst this broad range of usable materials, biopolymers play an important role in biotechnological, biomedical, and other applications. Biopolymers like gelatin, however, need to be crosslinked for most applications.
- nanofiber blends
- water-solubility
- polymer complex
- polymer blend
- crosslinker
- genipin
- aldehydes
- UV-crosslinking
- electron beam
- photo initiator
1. Introduction
Gelatin is derived from collagen by partial hydrolysis, either by acid processing (type A) or by alkaline or lime processing (type B) [1]. Gelatin is often processed from waste, e.g., from fish skin and bones after filleting [2]. As a natural material, gelatin shows different physical and chemical properties, depending on its origin [3][4][5][3,4,5]. Values typically measured in comparative studies are, e.g., the molecular weight, amino acid composition, gel strength, viscosity, melting point, as well as breaking force, water vapor permeability, or water solubility.
Gelatin’s physical and chemical properties as well as the abundant availability make it interesting for diverse applications in food, cosmetics, and the pharmaceutical industry, but also as a coating of photographic films. Other important areas of application are biotechnology and biomedicine. Especially gelatin-based porous scaffolds are of high interest in tissue engineering [6]. However, here, a challenge occurs due to the water-solubility of gelatin, which necessitates crosslinking after producing the scaffolds. There are different physical methods, such as UV irradiation or dehydrothermal treatment [7][8][7,8], chemical methods such as exposure to glutaraldehyde in the form of vapor or liquid [9][10][9,10] or to ethylcarbodiimidehydrochlorine (EDC) combined with N-hydroxysuccinimide (NHS) [11][12][11,12], or enzymatic treatment by genipin or transglutaminases [13][14][13,14]. Generally, the crosslinking method influences the physical and chemical properties of the resulting scaffold [15][16][17][15,16,17].
This is especially important for nanofibrous scaffolds, as they are often produced by electrospinning. The structure of these ultrathin fibers is usually modified by the crosslinking process, in this way also modifying the porosity, mechanical properties, and suitability for cell growth.
2. Electrospun Gelatin Nanofibers—Production and Properties
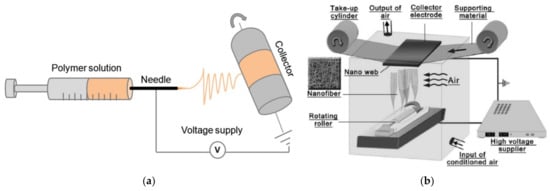
Electrospinning can be used to produce nanofiber mats by a relatively simple technique. In brief, a polymer solution or melt is introduced into an electric field that is produced by a high voltage between two electrodes, where the polymer solution or melt is accelerated to the counter electrode and in this way stretched until ultrathin fibers are deposited on the substrate, which usually shields the counter electrode [18][19][20][18,19,20]. Often used techniques are needle-based electrospinning (Figure 1a) [21] and wire-based electrospinning (Figure 1b) [22], while there are diverse other technologies available in the broad range of needleless electrospinning.
Electrospinning gelatin is in principle possible from aqueous solutions. For example, Zhang et al. investigated the influence of the spinning temperature as well as of the solid content in the solution on the morphology of the gelatin nanofiber mat [23]. They dissolved 30–40% gelatin with a molecular weight of 25 kDa in distilled water of 40 °C, varied the temperature of the solution in the syringe during spinning between 30 and 50 °C, and found that the viscosity decreased with temperature and increased with solid content, while the conductivity increased with temperature and with concentration. The average fiber diameter slightly decreased with temperature and strongly increased with gelatin concentration. Crosslinking was performed with EDC/NHS in a ratio of 2.5:1, resulting in reduced weight loss, significantly reduced swelling, and clearly modified stress–strain curves, which also depended strongly on whether they were measured in the dry or in the wet state.
3. Physical Crosslinking
One of the possibilities to crosslink gelatin nanofibers is based on the use of high-energetic light, i.e., UV irradiation. In this approach, inter- and intramolecular photodimerization techniques are used to control the crosslinking density [24][29]. For example, Ko et al. firstly prepared trans-cinnamic acid modified gelatin, used it for electrospinning from HFP with a needle-based technique on a rotating collector, and crosslinked these gelatin nanofibers by UV irradiation at 254 nm for different durations, before the unreacted gelatin was rinsed with water [24][29]. They found higher UV absorbance with increasing amount of cinnamoyl groups, with no absorbance of natural gelatin at the investigated wavelength of 270 nm. The crosslinking density also increased with increasing UV irradiation duration up to 3 h, while longer times did not show further crosslinking effects. However, comparison with genipin crosslinked gelatin nanofibers showed higher cell proliferation of the latter.4. Chemical Crosslinking
The most often used chemical crosslinking agents belong to the aldehydes. In many studies, glutaraldehyde is used to crosslink electrospun gelatin nanofibers, either as vapor or in fluid form [25][26][27][28][29][52,53,54,55,56]. On the one hand, GA is a very efficient crosslinking agent, as visible in Figure 2 [26][53]. On the other hand, glutaraldehyde is toxic, with highly problematic effects on the human health, such as chronic bronchitis and even possible genetic activity, reported in diverse studies [30][31][32][57,58,59]. Thus, different crosslinking chemicals are necessary, combining low-toxicity with efficient crosslinking.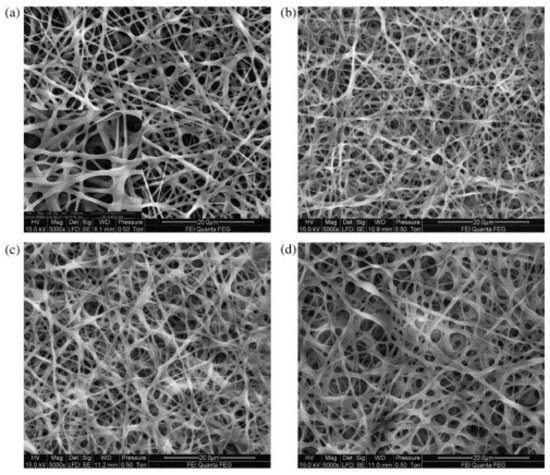
5. Enzymatic Crosslinking
Enzymatic crosslinking of gelatin is most often performed by genipin [34][35][36][100,101,102] or microbial transglutaminase [37][38][39][103,104,105]. Both are not non-toxic, but they are briefly mentioned here as examples for enzymatic crosslinking. Genipin is a molecule extracted from the gardenia plant fruit, which crosslinks gelatin in a two-step process, firstly by building heterocyclic linking of genipin to an amine in gelatin, followed by a nucleophilic substitution of the ester group in genipin resulting in covalent crosslinks between the primary amine residues [35][101]. Microbial transglutaminase (MTG) is a transferase that is assumed to build covalent bonds between different polymer chains, resulting in a hydrogel network [39][105]. Other enzymes that have been used to crosslink biopolymers belong to the transferases, hydrolases, and oxidoreductases [39][105], and they have been scarcely investigated for their possible use with electrospun nanofiber mats.6. Blending Gelatin with Other Polymers
As a last method to improve the mechanical strength and to reduce the water solubility of electrospun gelatin nanofiber mats, they can be blended with other polymers, in this way often retaining the positive properties of gelatin for tissue engineering in combination with the mechanical stability of the water-stable polymer. One of the typical blend partners of gelatin is chitosan. Chitosan is derived from chitin by partial deacetylation, and it is, similar to gelatin, biocompatible, biodegradable, and antimicrobial [40][115]. Dhandayuthapani et al. prepared chitosan/gelatin blend nanofibers by electrospinning from TFA/dichloromethane solution and found significantly better tensile strength, in the range of normal human skin, for the blended nanofibers [41][116]. Similarly, Yin-Guibo et al. found improved mechanical properties for silk fibroin/gelatin blended electrospun nanofiber mats, as compared to both pure materials [42][117]. The solubility in water was not mentioned in these studies.7. Biomedical Applications of Crosslinked Gelatin Nanofiber Mats
In addition to the aforementioned possible applications of crosslinked gelatin nanofibers mats for tissue engineering, cell growth, wound healing, and other biomedical applications, this section gives some more examples of these applications without the previous restriction to non-toxic crosslinking methods.
Jalaja et al. used crosslinking with dextran aldehyde to improve thermal and mechanical properties as well as the structural integrity in an aqueous solution [43][128]. The nanofiber mat was found to be non-cytotoxic for mouse subcutaneous fibroblasts L-929 and showed significantly higher cell proliferation as compared to glutaraldehyde crosslinked nanofiber mats.
Using GT vapor crosslinking, Gui et al. compared human mesenchymal stem cell growth on randomly oriented and highly aligned gelatin nanofiber mats and found more effective proliferation on the oriented fibers [44][129]. Padrao et al. also used GA to crosslink electrospun gelatin nanofiber mats and found no inhibition of MC-3T3-E1 cell adhesion and proliferation in randomly oriented and aligned gelatin nanofiber mats [45][130]. With genipin crosslinking of gelatin nanofiber mats, Gualandi found no cytotoxicity against human primary chondrocytes, but oppositely the promotion of chondrocyte viability and differentiation [46][109]. Chen et al. also found cell compatibility for mouse mesangial cells grown on GA-crosslinked gelatin nanofibers [29][56].
Luo et al. compared gelatin nanofiber mats crosslinked with glutaraldehyde, genipin, and EDC/NHS and found that they all supported adhesion, spreading, and proliferation of MC-3T3-E1 cells, with a lower cell viability after GA crosslinking, while similar values were found for genipin and EDC/NHS [47][131].
Ghassemi and Slaughter used DHT and EDC/NHS crosslinking on gelatin nanofiber mats and suggested EDC/NHS crosslinked electrospun gelatin nanofiber mats as scaffolds for cell-based assays [48][37].
Hivechi et al. used NIH/3T3 mouse fibroblasts to investigate the influence of adding cellulose nanocrystals to electrospun gelatin nanofibers [49][132]. They found a slightly increased biodegradability of the fibers crosslinked by GA vapor and no effect on the cytotoxicity, cell growth, and proliferation.
Using GA vapor crosslinking, Laha et al. used gelatin nanofiber mats for oral drug delivery with piperine as a model drug [25][50][52,133]. By modulating the pH value of the release medium and the crosslinking efficiency, they could tailor the piperine release rate.
Del Gaudio et al. also used genipin-crosslinked electrospun gelatin nanofiber mats to investigate loading with human vascular endothelial growth factor (VEGF) and long-term release of VEGF [51][108]. Human mesenchymal stromal cells growing on these nanofiber mats showed higher cell viability and endothelial differentiation when growing on these VEGF-loaded nanofiber mats, with the bioactive and pro-angiogenic potential kept for two weeks, showing that VEGF-loaded genipin-crosslinked gelatin nanofiber mats may be suitable for the stimulation of angiogenesis in tissue engineering.
Bactericidal wound dressing was produced by adding poly([2-(methacryloyloxy)ethyl] trimethylammonium chloride) (PMETAC) to the gelatin electrospinning solution and crosslinking the obtained nanofiber mats with GA vapor [52][134]. Inal and Mülazimoglu showed good cell attachment and proliferation and suggested using these antimicrobial, biocompatible nanofiber mats as wound dressing.
Especially for postoperative surgical wounds, Rath et al. suggested gelatin nanofiber mats loaded with cefazolin/zinc oxide nanoparticles as antimicrobial wound dressings [53][135].
As these few examples show, in addition to those given in the previous sections, the main applications of crosslinked gelatin nanofiber mats in biomedicine are related to tissue engineering and cell growth, but also for wound healing with tailorable drug delivery.
