As a traditional source for modern pharmaceutical discovery and potential drug leads, natural products have played an integral role in treating patients due to their unique structural, chemical, and biological diversity. A wide range of natural products can be considered promising anti-COVID-19 or anti-lung cancer agents have gained widespread attention, including natural products as monotherapy for the treatment of SARS-CoV-2 (ginkgolic acid, shiraiachrome A, resveratrol, and baicalein) or lung cancer (daurisoline, graveospene A, deguelin, and erianin) or in combination with FDA-approved anti-SARS-CoV-2 agents (cepharanthine plus nelfinavir, linoleic acid plus remdesivir) and anti-lung cancer agents (curcumin and cisplatin, celastrol and gefitinib).
- natural product
- SARS-CoV-2
- lung cancer
- natural remedy
1. Natural Products as Monotherapy for the Treatment of SARS-CoV-2
Natural products have demonstrated potential value, which supports this strategy as an indispensable research focus in the fight against the COVID-19 epidemic [1][2]. The chemical structures of the components described in this section are shown in
pro
pro), has a vital function in viral replication and is, therefore, a preferred drug target [3]. The papain-like protease (PL
pro), another prime therapeutic target, plays an essential role in maturing viral RNA polyproteins and dysregulation of host inflammation [4]. Ginkgolic acid, a phenolic acid, is an essential component of the traditional herbal medicine
Ginkgo biloba (EGb) [5]. A study has demonstrated that ginkgolic acid is characterized by half-maximal inhibitory concentration (IC
50
pro
pro, respectively [6]. The study unambiguously showed that ginkgolic acid exerts good dual-inhibitory effects through its irreversible binding to SARS-CoV-2 cysteine proteases [6].
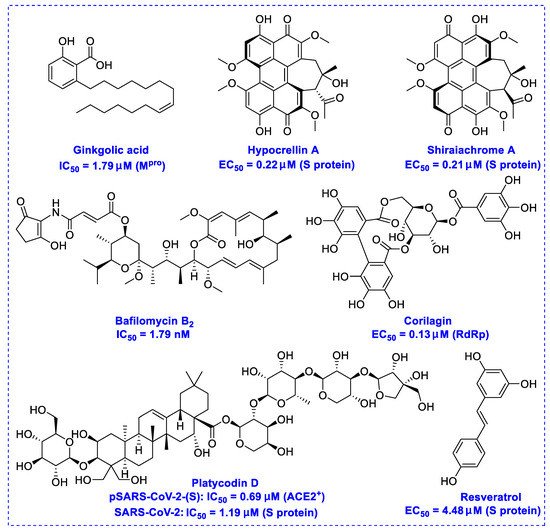
Figure 1.
Angiotensin-converting enzyme 2 (ACE2), an essential ingredient of the renin–angiotensin–aldosterone system (RAAS), is a critical host cell surface receptor for viral infection [7]. The glycosylated spike protein (S protein) plays an essential role in mediating viral entry via interactions with the ACE2 cell surface receptor [8]. Hypocrellin A and shiraiachrome A, two-axial chiral perylenequinones, have been reported to exhibit potent effects on the infected monkey Vero E6 cell line by inhibiting the activity of the SARS-CoV-2 S protein at EC
50 values of 0.22 μM and 0.21 μM, respectively, while at doses of up to 10 μM, these presented no observable cytotoxicity against these cells [9].
Transmembrane protease serine 2 (TMPRSS2), a critical factor enabling SARS-CoV-2 infection, can interact with ACE2 [10]. It has been reported that platycodin D, a triterpenoid saponin isolated from
Platycodon grandiflorum, prevents TMPRSS2-driven infection in vitro by impairing membrane fusion [11]. Platycodin D has IC
50
+
+
50 values of 1.19 μM and 4.76 μM for SARS-CoV-2 in TMPRSS2-negative Vero cells and TMPRSS2-positive Calu-3 cells, respectively [11]. Resveratrol, a remarkable phytoalexin, may effectively inhibit the replication of SARS-CoV-2 S protein in Vero E6 cells at an EC
50 of 4.48 μM [12], and has an excellent safety tracking record, with no cytotoxicity even up to a concentration of 150 µM [13].
The RNA-dependent RNA polymerase (RdRp) of SARS-CoV-2 is another promising target that regulates the replication of the viral genome [14]. Corilagin, a non-nucleoside inhibitor, is a gallotannin isolated from the medicinal plant
Phmllanthi Fructus [15]. Corilagin has been reported to inhibit SARS-CoV-2 infection with an EC
50 value of 0.13 μM in a concentration-dependent manner by preventing the conformational change of RdRp and inhibits SARS-CoV-2 replication [16]. Furthermore, corilagin, as identified via molecular dynamics simulation-guided studies, could also be used as an endogenous M
pro
pro activity at concentrations of 20 μM in vitro [17].
2
Streptomyces
50 values of 5.11 nM (in the full-time approach) and 8.32 nM (in the pretreatment-of-virus approach) in Vero E6 cells, respectively [18]. While bafilomycin B
2
Table 1.
| No. | Name | Structure | EC50 or IC50 (μM) | Strain | Refs |
|---|
| 1 | Acetoside | 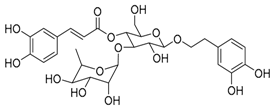 |
0.043 | Vero E6 cells | [19][39] |
| 2 | Anacardic acid | 2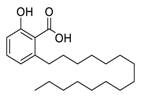 |
2.07 | USA-WA1/2020 | [6][26] |
| Asiatic acid |  |
Inhibited the ionizing radiation-induced migration and invasion | [ | 69][89] | 3 |
| 3 | Baicalein | 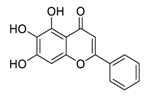 |
Restrained ezrin tension by decreasing inducible nitric oxide synthase expression levels, suppress invasion, reduced vasculogenic mimicry formation | [70][71][72][90,91,92] | |
| 4 | Baicalin | 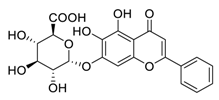 |
Inhibited the invasion, migration, angiogenesis, and Akt/mTOR pathway | [73][74][ | |
| 37 | |||||
| ] | |||||
| [ | |||||
| 38 | |||||
| ] | |||||
| [ | |||||
| 57 | |||||
| , | |||||
| 58 | |||||
| ] | |||||
| 93 | , | 94 | ] | ||
| 5 | Casticin | 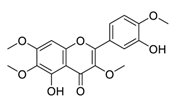 |
Induced the expressions and nuclear translocation of phosphorylation of H2AX | [75][95] | |
| 6 | Dioscin | 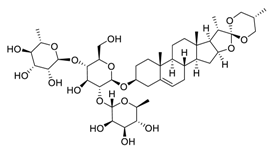 |
Down-regulated signal transducer and activator of transcription 3 and c-Jun N-terminal kinase signaling pathways | [76][96] | |
| 7 | EGCG | 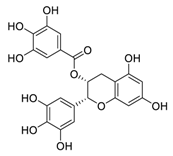 |
Regulated CTR1 expression through the ERK1/2/NEAT1 signaling pathway | [77][78][97,98] | |
| 21 | Naringenin | 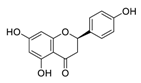 |
0.092 | Vero E6 cells | [19][39][39,59] |
| 22 | Osajin | 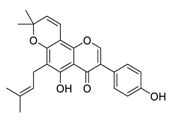 |
3.87 | Vero E6 cells | [40][60] |
| 23 | 2,3′,4,5′,6-Pentahydroxybenzophenone | 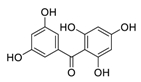 |
0.102 | Vero E6 cells | [19][39] |
| 24 | Procyanidin B2 | 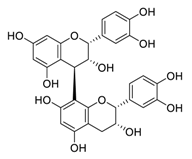 |
75.3 | Vero E6 cells | [27][47] |
| 25 | Punicalagin | 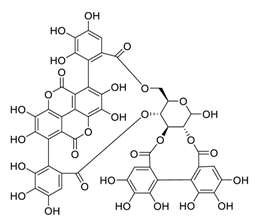 |
7.20 | Vero E6 cells | [28][48] |
| 26 | Sennoside B | 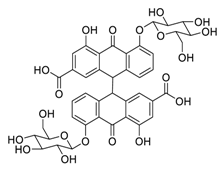 |
0.104 | Vero E6 cells | [19][39] |
| 27 | Shikonin | 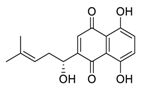 |
15.75 | Vero E6 cells | [41][61] |
| 28 | Δ9-Tetrahydrocannabinol |  |
10.25 | Vero E6 cells | [25][45] |
| 29 | Tetrandrine |  |
3.00 | Vero E6 cells | [40][60] |
| 30 | Theaflavin |  |
8.44 | HEK293T human embryonic kidney cells |
[42][62] |
Traditional Chinese medicines have attracted considerable attention due to their ability to effectively inhibit SARS-CoV-2 [43][44][45]. For example, the Qingfei Paidu decoction (QFPD) has shown an ability to treat COVID-19 patients at all stages with excellent clinical efficacy (cure rate >90%) [46][47]. Shuanghuanglian oral liquid or injection (SHL), another well-known traditional Chinese medicine, dose-dependently inhibits SARS-CoV-2 M
pro replication [48]. In addition to the above-mentioned QFPD and SHL, several other traditional Chinese medicines (such as Kegan Liyan oral liquid and Toujie Quwen granule) listed in
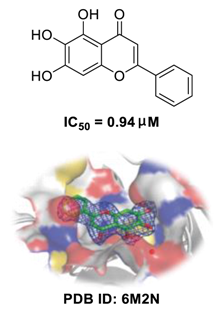
Scutellaria baicalensis
50 of 0.94 μM, and SI > 212) [49]. Furthermore, it is crucial to investigate how herbal medicine affects SARS-CoV-2 infection by studying its active ingredients. To elucidate the underlying molecular mechanisms, a crystal structure of SARS-CoV-2 M
pro complexed with baicalein was constructed at a resolution of 2.2 Å (the Protein Data Bank (PDB) ID: 6M2N) [48]. Analysis of the core of the substrate-binding pocket revealed multiple interactions (such as hydrogen bonding with Leu141/Gly143 and Ser144/His163, π–π interactions with Cys145 and His4, and hydrophobic interactions with Met49 and His41), which effectively blocked SARS-CoV-2 replication via noncovalent incorporation [48]. The relevant studies [50][51][52] provided direct data for a better understanding of the molecular mechanisms of Chinese herbal medicine by studying its active ingredients.
Table 2.
| Baicalein (The Active Ingredient of Huangqin) | Molecular Mechanisms of Baicalein | Herbal Formula Containing Huangqin | Registration Number | Sample Size of the Control Group |
|---|
 |
RdRp inhibitor via noncovalent incorporation [53][73], potent antagonists against TMPRSS2 [50][70], improving respiratory function, decreasing IL-1β and TNF-α levels, and inhibiting cell infiltration [51][52][71,72]. | Qingfei Paidu decoction | ChiCTR2000029433 | 120 | ||||
| ChiCTR2000030883 | 100 | |||||||
| ChiCTR2000032767 | Andrographolide | 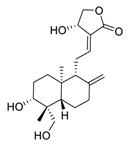 |
0.034 | Calu-3 cells | [20][21][40, | 78241] | ||
| 4 | Apigenin-7-O-glucoside | 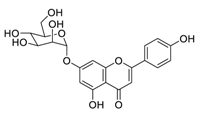 |
0.074 | Vero E6 cells | [19][39 | |||
| Xinguan I decoction | ] | |||||||
| ChiCTR2000029637 | 50 | 5 | Artemisinin |  |
64.45 | Vero E6 cells | [21][22][41 | |
| Tanreqing capsules | ChiCTR2000029813 | , | 36 | 42 | ] | |||
| 6 | Azithromycin |  |
2.12 | Caco-2 cells | [23][43] | |||
| Tanreqing injection | ChiCTR2000029432 | 72 | 7 | Baicalin | 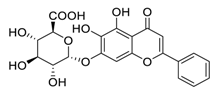 |
7.98 | Vero E6 cells | [24][44] |
| Kegan Liyan oral liquid | ChiCTR2000033720 | 240 | 8 | Cannabidiol | 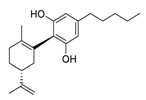 |
7.91 | Vero E6 cells | [25][26][45,46] |
| ChiCTR2000033745 | 240 | 9 | Catechin-3-O-gallate | 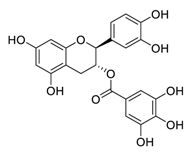 |
2.98 | Vero E6 cells | ||
| ChiCTR2000031982 | [ | 27 | 240 | ] | [47] | |||
| 10 | Chebulagic acid | 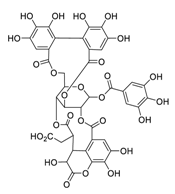 |
9.76 | Vero E6 cells | ||||
| Shuanghuanglian oral liquid | [ | 28 | ] | [ | 48] | |||
| ChiCTR2000033133 | 11 | Daurisoline | 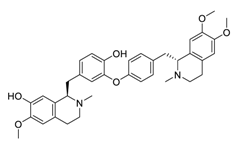 |
3.66 | Vero E6 cells | [29][49] | ||
| 30 | ||||||||
| ChiCTR2000029605 | 100 | 12 | EGCG | 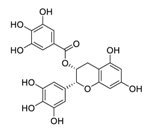 |
0.874 | Vero E6 cells | [24][30][44,50] | |
| Toujie Quwen granule | ChiCTR2000031888 | 150 | 13 | Emetine |  |
0.46 | Vero E6 cells | [31][32][51,52] |
| 14 | Epicatechin-3-O-gallate |  |
5.21 | Vero E6 cells | [27][47] | |||
| 15 | Gallinamide A | 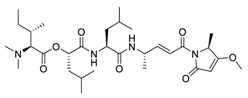 |
0.028 | Vero E6 cells | [33][53] | |||
| 16 | Gallocatechin-3-O-gallate | 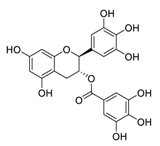 |
6.38 | Vero E6 cells | [27][47] | |||
| 17 | Hopeaphenol | 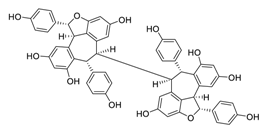 |
2.3 | B.1.351 | [34][54] | |||
| 18 | Ipomoeassin F | 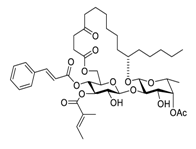 |
semi-permeabilized mammalian cells | [35][55] | ||||
| 19 | Kobophenol A | 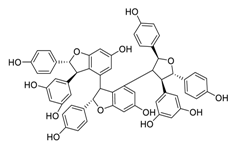 |
1.81 | Vero E6 cells | [36][56] | |||
| 20 | Myricetin | 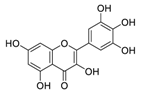 |
0.22 | Vero E6 cells | [ |
2. Natural Products as Monotherapy for the Treatment of Lung Cancer
Nelumbo nucifera Gaertn [54]. The chemical structures of the molecules discussed in this section are shown in
Figure 2. Daurisoline increases the degradation of β-catenin by targeting heat shock protein 90 (HSP90) directly and decreases the expression of MYC proto-oncogene (c-MYC) and cyclin D1, which resulted in cell cycle arrest at the G1 phase in human lung cancer A549 cells and Hop62 cells lines to exert its anti-lung cancer activity [55]. More importantly, in animals, daurisoline has been reported to be a promising anti-lung cancer agent (by inhibiting tumor growth in lung cancer xenografts) with no observable side effects, thus highlighting a potential role for daurisoline in the treatment of lung cancer [55]. Another recent study has shown that daurisoline can effectively inhibit SARS-CoV-2 replication at IC
50 values of 3.664 μM and 0.875 μM in Vero E6 cells and in human pulmonary alveolar epithelial cells (HPAEpiC), respectively [29].
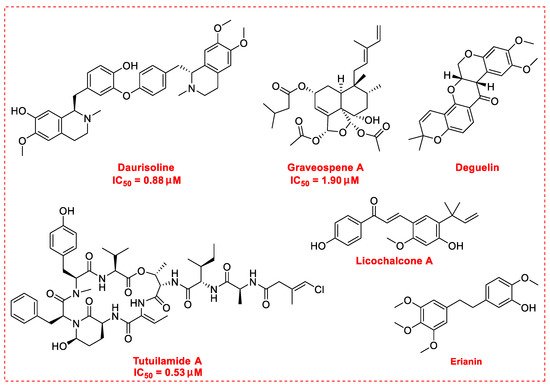
Figure 2.
Casearia graveolens
50 value of 1.9 μM by inducing cell cycle arrest in phase G0/G1 [56]. Deguelin, a protein kinase B (AKT) kinase inhibitor, is isolated from the African plant
Mundulea sericea (Leguminosae) and is commonly used to inhibit the growth of several types of human cancer cell lines [57]. Deguelin promoted the phosphorylation of myeloid cell leukemia sequence-1 (Mcl-1) protein and induced the inhibition of the wildtype and mutated epidermal growth factor receptor (EGFR)-Akt signaling pathway, which resulted in activation of downstream GSK3β/FBW7 and profound anti-NSCLC activity with no obvious side effects in vivo [58].
Xinjiang licorice
Glycyrrhiza inflata. Licochalcone A is known to possess a broad spectrum of activities with important pharmacological effects in various cancer cell lines [59]. Licochalcone A can significantly increase autophagic cytotoxicity (in both A549 and H460 cell lines) and downregulated the expression of c-IAP1, c-IAP2, XIAP, survivin, c-FLIPL, and RIP1, apoptosis-related proteins via inhibiting the activity of phosphorylated extracellular signal-regulated kinase (ERK) and autophagy [60]. In addition, licochalcone A has been reported to abolish the expression of programmed death ligand-1 (PD-L1) by increasing reactive oxygen species (ROS) levels in a time-dependent manner and interfering with protein translation in cancer cells [61]. Further, licochalcone A can inhibit PD-L1 translation likely through the inhibition of the phosphorylation of 4EBP1 and activation of the PERK-eIF2α signaling pathway [61]. Licochalcone A plays a vital role in reversing the ectopic expression of key microRNA (miR-144-3p, miR-20a-5p, miR-29c-3p, let-7d-3p, and miR-328-3p) to elicit lung cancer chemopreventive activities both in vivo and in vitro [62]. In addition, licochalcone A has been reported to inhibit EGFR signaling and reduced the expression of Survivin protein in a cap-dependent translation manner to exhibit profound activity in mutated NSCLC cells [63].
Dendrobium chrysotoxum Lindl and has been proposed as an apoptosis-inducing agent in human lung cancer cells [64]. The main mechanisms of its anti-lung cancer activity involve the induction of ferroptosis by activating Ca
2+/calmodulin signaling, inhibition of cell proliferation and metastasis, and induction of cell cycle arrest in phase G2/M [65].
Schizothrix
50 value of 0.53 μM [66]. Tutuilamide A, with the help of the vinyl chloride side chain, showed enhanced inhibitory potency with high selectivity (IC
50 0.73 nM) for human neutrophil elastase, which is associated mainly with the migration and metastasis of lung cancer cells [67]. Besides the above-mentioned molecules,
Table 3.
| No. | Name | Structure | Mechanism of Anti-Lung Cancer | Refs |
|---|
| 1 | Acovenoside A | 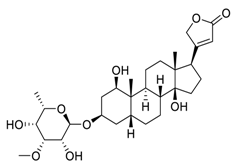 |
Inhibit the adenosine triphosphate (ATP)-dependent Na+/K+ exchange through the Na+/K+-ATPase | [68][88] |
| 8 | ||||
| Ellagic acid | ||||
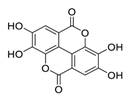 | ||||
| Inhibited tumor growth, increased p-AMPK, and suppressed hypoxia-inducible factor 1α levels | [ | 79 | ] | [99] |
| 9 | Erianthridin | 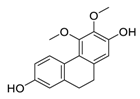 |
Attenuated extracellular signal-regulated kinase activity and mediated apoptosis, matrix-degrading metalloproteinases (MMPs) expression | [80][81][100,101] |
| 10 | Eugenol | 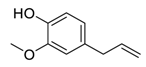 |
Restriction of β-catenin nuclear transportation | [82][102] |
| 11 | Formononetin | 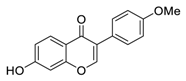 |
Inhibited EGFR-Akt signaling, which in turn activates GSK3β and promotes Mcl-1 phosphorylation in NSCLC cells | [83][84][103,104] |
| 12 | Gallic Acid | 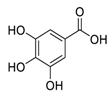 |
Inhibited of EGFR activation and impairment, inhibition of phosphoinositide 3-kinase (PI3K) and AKT phosphorylation | [85][86][105,106] |
| 13 | Glochidiol | 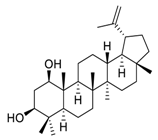 |
Inhibited tubulin polymerization | [87][107] |
| 14 | Gracillin | 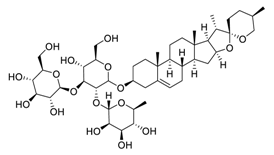 |
Inhibited both glycolysis and mitochondria-mediated bioenergetics, induced apoptosis through the mitochondrial pathway | [88][89][108,109] |
| 15 | Hispidulin | 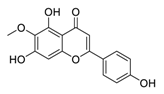 |
Promoted apoptosis by hispidulin via increased generation of ROS | [90][110] |
| 16 | Icaritin | 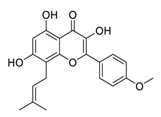 |
Downregulated the immunosuppressive cytokine (TNF-α, IL10, IL6) and upregulated chemotaxis (CXCL9 and CXCL10) | [91][111] |
| 17 | Isoharringtonine | 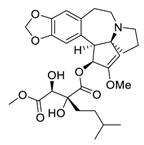 |
Induced death tumor spheroids by activating the intrinsic apoptosis pathway | [92][112] |
| 18 | Kaempferol | 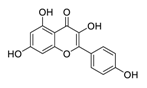 |
Inhibitor of nuclear factor erythroid 2-related factor 2 | [93][113] |
| 19 | Liriopesides B | 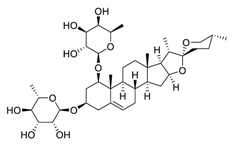 |
Reduced proliferation, and induced apoptosis and cell cycle arrest, inhibited the progression of the cell cycle from the G1 to the S phase | [94][114] |
| 20 | Nagilactone E | 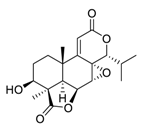 |
Activated the c-Jun N-terminal kinases, increased the phosphorylation, and promoted the localization of c-Jun in the nucleus | [95][96][115,116] |
| 21 | 8-Oxo-epiberberine | 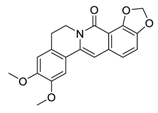 |
Inhibited TGF-β1-induced epithelial-mesenchymal transition (EMT) possibly by interfering with Smad3 | [97][117] |
| 22 | Parthenolide | 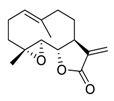 |
Reduced the phosphorylation of EGFR and downstream signaling pathways mitogen-activated protein kinase (MAPK)/ERK, inhibited PI3K/Akt/FoxO3α signaling | [98][99][100][118,119,120] |
| 23 | PDB-1 | 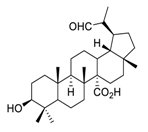 |
Suppressed lung cancer cell migration and invasion via FAK/Src and MAPK signaling pathways | [101][121] |
| 24 | Polyphyllin I | 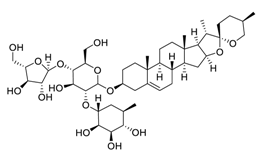 |
Induced autophagy by activating AMPK and then inhibited mTOR signaling, promoted apoptosis, modulated the PI3K/Akt signaling | [102][103][122,123] |
| 25 | Quercetin | 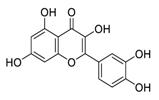 |
Inhibited proliferation and induced apoptosis | [104][124] |
| 26 | Silibinin | 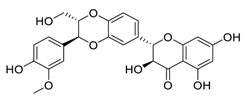 |
Inhibited cell proliferation, migration, invasion, and EMT expression | [105][125] |
| 27 | Sinomenine | 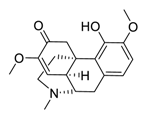 |
Downregulated expression of MMPs and miR-21, suppressed α7 nicotinic acetylcholine receptors expression | [106][107][108][126,127,128] |
| 28 | Toxicarioside O | 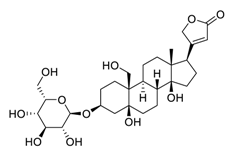 |
Decreased the expression of trophoblast cell surface antigen 2, resulting in inhibition of the PI3K/Akt pathway and EMT program | [109][129] |
| 29 | Vincamine | 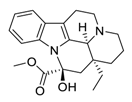 |
Interaction with the apoptotic protein caspase-3 | [110][130] |
| 30 | Xanthohumol | 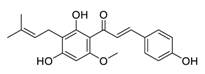 |
Suppressed ERK1/2 signaling and reduced the protein levels of FOS-related antigen 1, decreased the mRNA level of cyclin D1 | [111][131] |
