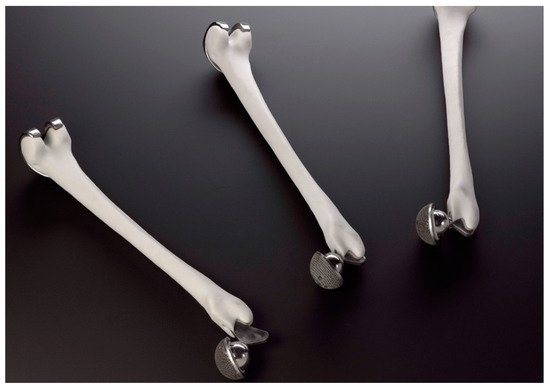
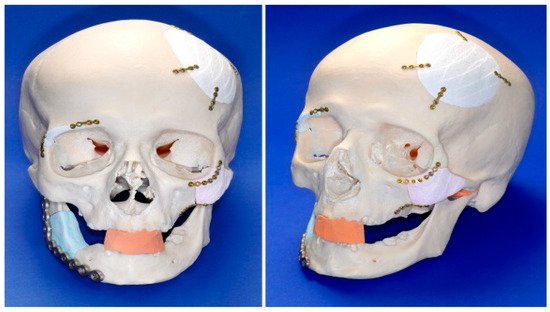
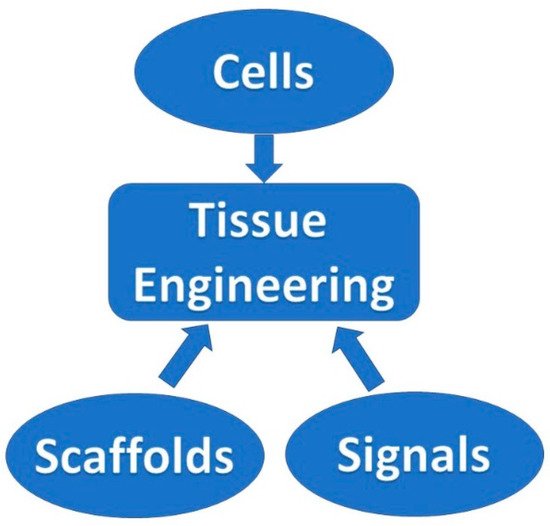
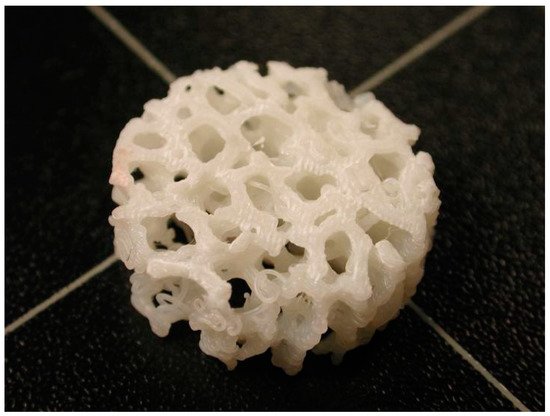
Over time, the fabrication of lattice, porous structures has always been a controversial field for researchers and practitioners. Such structures could be fabricated in a stochastic way, thus, with limited control over the actual porosity percentage. The emerging technology of 3D printing, offered an automated process that did not require the presence of molds and operated on a layer-by-layer deposition basis, provided the ability to fabricate almost any shape through a variety of materials and methods under the umbrella of the ASTM terminology “additive manufacturing”. In the field of biomedical engineering, the technology was embraced and adopted for relevant applications, offering an elevated degree of design freedom. Applications range in the cases where custom-shaped, patient-specific items have to be produced. Scaffold structures were already a field under research when 3D printing was introduced. These structures had to act as biocompatible, bioresorbable and biodegradable substrates, where the human cells could attach and proliferate. In this way, tissue could be regenerated inside the human body. One of the most important criteria for such a structure to fulfil is the case-specific internal geometry design with a controlled porosity percentage. 3D printing technology offered the ability to tune the internal porosity percentage with great accuracy, along with the ability to fabricate any internal design pattern.
- 3D printing
- lattice structures
- tissue engineering
- regenerative medicine
- scaffold
Note: The following contents are extract from your paper. The entry will be online only after author check and submit it.
1. Introduction
2. Scaffold Structures Modus Operandi Criteria
where Vstr stands for the volume of the struts and Vcub stands for the overall volume of the scaffold.
Scaffold Desired Mechanical Behavior Characteristics
3. The Potential of 3D-Printed Scaffold Structures
| 3D Printing Techniques | Materials | Strategy/Printing Process | |
|---|---|---|---|
| Acellular Scaffolds | Powder Bed Fusion (PBF) (SLM/SLS/EBM) |
Titanium/Aluminum Cobalt-Chromium-Molybdenum alloys in powder form [41] |
Layers of powder thermally fused by an energy source (laser or electron beam) |
| Direct Energy Deposition (DED) | Metal alloys in powder or wire form (titanium/aluminum alloys, refractory metals such as tantalum, tungsten, niobium) | The feedstock material is forwarded through the nozzle, where it is melted by a focused heat source (laser or electron beam) and deposited on the build platform. Both nozzle and heat source are attached on a robotic arm or a gantry system | |
| Fused Deposition Modelling (FDM) | Biodegradable and biocompatible polymers in filament form such as PCL etc. [42][75] | The filament material is forwarded to the extrusion nozzle where it is heated and melted. It is then deposited on a build platform enclosed in a heated chamber | |
| Cellular Scaffolds | Extrusion Based Bioprinting (EBB) | Hydrogel solutions, bio-ink materials [43] | Bio-ink material is dispensed with high precision resulting in targeted cell deposition. Cells are encapsulated in cylindrical filaments forming pre-determined 3D structures |
| Laser Based Bioprinting * (LBB) | Cells of various types, culture medium [76] | A laser beam focused through a low numerical value aperture lens, resulting in the deposition of cells through culture media on pre-designated spots on a glass surface | |
| Inkjet Based Bioprinting * (DNA and protein printing) | Hydrogels (Alginate, PEG, Alkanethiols etc.), binders (acrylic ink, phosphoric acid, PVA etc.) Polymers (PCL, PLA, PLGA etc.) dissolved or dispersed in organic solvents [44][77] | Modified commercial inkjet printers that deposit bio-ink material that forms self-assembled layers | |
| Cell inkjet Bioprinting * | Cells of various types, bio-paper [44] | Direct deposition of cells using printheads on a substrate | |
| Microvalve-based bioprinting * | Hydrogels of specific viscosity [45] | The process uses a platform and multiple electromechanical micro-valve printheads depositing bio-ink | |
| VAT Polymerization | Bio-resins including PEGDA and GelMA etc. [46] | Specific wavelength laser is emitted in the bio-resins achieving its curing via photopolymerization processes | |
| 3D Printing Techniques | Materials | Strategy/Printing Process | |
| Acellular Scaffolds | Powder Bed Fusion (PBF) (SLM/SLS/EBM) |
Titanium/Aluminum Cobalt-Chromium-Molybdenum alloys in powder form [41] |
Layers of powder thermally fused by an energy source (laser or electron beam) |
| Direct Energy Deposition (DED) | Metal alloys in powder or wire form (titanium/aluminum alloys, refractory metals such as tantalum, tungsten, niobium) | The feedstock material is forwarded through the nozzle, where it is melted by a focused heat source (laser or electron beam) and deposited on the build platform. Both nozzle and heat source are attached on a robotic arm or a gantry system | |
| Fused Deposition Modelling (FDM) | Biodegradable and biocompatible polymers in filament form such as PCL etc. [42,75] | The filament material is forwarded to the extrusion nozzle where it is heated and melted. It is then deposited on a build platform enclosed in a heated chamber | |
| Cellular Scaffolds | Extrusion Based Bioprinting (EBB) | Hydrogel solutions, bio-ink materials [43] | Bio-ink material is dispensed with high precision resulting in targeted cell deposition. Cells are encapsulated in cylindrical filaments forming pre-determined 3D structures |
| Laser Based Bioprinting * (LBB) | Cells of various types, culture medium [76] | A laser beam focused through a low numerical value aperture lens, resulting in the deposition of cells through culture media on pre-designated spots on a glass surface | |
| Inkjet Based Bioprinting * (DNA and protein printing) | Hydrogels (Alginate, PEG, Alkanethiols etc.), binders (acrylic ink, phosphoric acid, PVA etc.) Polymers (PCL, PLA, PLGA etc.) dissolved or dispersed in organic solvents [44,77] | Modified commercial inkjet printers that deposit bio-ink material that forms self-assembled layers | |
| Cell inkjet Bioprinting * | Cells of various types, bio-paper [44] | Direct deposition of cells using printheads on a substrate | |
| Microvalve-based bioprinting * | Hydrogels of specific viscosity [45] | The process uses a platform and multiple electromechanical micro-valve printheads depositing bio-ink | |
| VAT Polymerization | Bio-resins including PEGDA and GelMA etc. [46] | Specific wavelength laser is emitted in the bio-resins achieving its curing via photopolymerization processes |
References
- Kantaros, A.; Piromalis, D. Employing a Low-Cost Desktop 3D Printer: Challenges, and How to Overcome Them by Tuning Key Process Parameters. Int. J. Mech. Appl. 2021, 10, 11–19.
- Kantaros, A.; Giannatsis, J.; Karalekas, D. A novel strategy for the incorporation of optical sensors in Fused Deposition Modeling parts. In Proceedings of the International Conference on Advanced Manufacturing Engineering and Technologies, Stockolm, Sweden, 27–30 October 2013; Universitets service US AB, KTH Royal Institite of Technology: Stockholm, Sweden, 2013. ISBN 978-91-7501-893-5.
- Kantaros, A.; Karalekas, D. FBG based in situ characterization of residual strains in FDM process. In Residual Stress, Thermomechanics & Infrared Imaging, Hybrid Techniques and Inverse Problems; Springer: Cham, Switzerland, 2014; Volume 8, pp. 333–337.
- Berman, B. 3-D printing: The new industrial revolution. Bus. Horizons 2012, 55, 155–162.
- Harrysson, O.L.; Cansizoglu, O.; Marcellin-Little, D.J.; Cormier, D.R.; West, H.A. Direct metal fabrication of titanium implants with tailored materials and mechanical properties using electron beam melting technology. Mater. Sci. Eng. C 2008, 28, 366–373.
- Bourell, D.L.; Leu, M.C.; Rosen, D.W. Roadmap for Additive Manufacturing Identifying the Future of Freeform Processing; The University of Texas at Austin Laboratory for Freeform Fabrication Advanced Manufacturing Center: Austin, TX, USA, 2009.
- Mazzoli, A.; Germani, M.; Raffaeli, R. Direct fabrication through electron beam melting technology of custom cranial implants designed in a PHANToM-based haptic environment. Mater. Des. 2009, 30, 3186–3192.
- Klammert, U.; Gbureck, U.; Vorndran, E.; Rödiger, J.; Meyer-Marcotty, P.; Kübler, A.C. 3D powder printed calcium phosphate implants for reconstruction of cranial and maxillofacial defects. J. Cranio-Maxillofacial Surg. 2010, 38, 565–570.
- Melchels, F.P.; Feijen, J.; Grijpma, D.W. A review on stereolithography and its applications in biomedical engineering. Biomaterials 2010, 31, 6121–6130.
- Puri, P.M.; Khajuria, H.; Nayak, B.P.; Badiye, A. Stereolithography: Potential Applications in Forensic Science. Res. J. Eng. Sci. 2012, 1, 47–50.
- Schrot, J.; Pietila, T.; Sahu, A. State of the art: 3D printing for creating compliant patient-specific congenital heart defect models. J. Cardiovasc. Magn. Reson. 2014, 16, W19.
- Guillotin, B.; Guillemot, F. Cell patterning technologies for organotypic tissue fabrication. Trends Biotechnol. 2011, 29, 183–190.
- Langer, R.; Vacanti, J.P. Tissue engineering. Science 1993, 260, 920–926.
- O’Brien, F.J. Biomaterials & scaffolds for tissue engineering. Mater. Today 2011, 14, 88–95.
- Vacanti, J.P.; Morse, M.A.; Saltzman, W.M.; Domb, A.J.; Perez-Atayde, A.; Langer, R. Selective cell transplantation using bioabsorbable artificial polymers as matrices. J. Pediatr. Surg. 1988, 23, 3–9.
- Wakitani, S.; Imoto, K.; Yamamoto, T.; Saito, M.; Murata, N.; Yoneda, M. Human autologous culture expanded bone marrow mesenchymal cell transplantation for repair of cartilage defects in osteoarthritic knees. Osteoarthr. Cartil. 2002, 10, 199–206.
- Ikada, Y. Challenges in tissue engineering. J. R. Soc. Interface 2006, 3, 589–601.
- Anthony Atala, Wake Forest Innovations. Available online: (accessed on 13 May 2021).
- Erwin, R. Facilitating 3D Organ Printing with Plant-Based BioInks, Pharma’s Almanac, PAP-Q4-19-CL-018. 2019. Available online: (accessed on 13 May 2021).
- Atala, A.; Bauer, S.B.; Soker, S.; Yoo, J.J.; Retik, A.B. Tissue-engineered autologous bladders for patients needing cystoplasty. Lancet 2006, 367, 1241–1246.
- Elliott, M.J.; De Coppi, P.; Speggiorin, S.; Roebuck, D.; Butler, C.R.; Samuel, E.; Crowley, C.; McLaren, C.; Fierens, A.; Vondrys, D.; et al. Stem-cell-based, tissue engineered tracheal replacement in a child: A 2-year follow-up study. Lancet 2012, 380, 994–1000.
- De Coppi, P.; Delo, D.; Farrugia, L.; Udompanyanan, K.; Yoo, J.J.; Nomi, M.; Atala, A.; Soker, S. Angiogenic Gene-Modified Muscle Cells for Enhancement of Tissue Formation. Tissue Eng. 2005, 11, 1034–1044.
- Dong, L.; Wang, S.-J.; Zhao, X.-R.; Zhu, Y.-F.; Yu-Fang, Z. 3D- Printed Poly(ε-caprolactone) Scaffold Integrated with Cell-laden Chitosan Hydrogels for Bone Tissue Engineering. Sci. Rep. 2017, 7, 1–9.
- Nicodemus, G.D.; Bryant, S.J. Cell Encapsulation in Biodegradable Hydrogels for Tissue Engineering Applications. Tissue Eng. Part B Rev. 2008, 14, 149–165.
- Kantaros, A.; Karalekas, D. Fiber Bragg grating based investigation of residual strains in ABS parts fabricated by fused deposition modeling process. Mater. Des. 2013, 50, 44–50.
- Moroni, L.; de Wijn, J.; van Blitterswijk, C. 3D fiber-deposited scaffolds for tissue engineering: Influence of pores geometry and architecture on dynamic mechanical properties. Biomaterials 2006, 27, 974–985.
- Amirkhani, S.; Bagheri, R.; Yazdi, A.Z. Effect of pore geometry and loading direction on deformation mechanism of rapid prototyped scaffolds. Acta Mater. 2012, 60, 2778–2789.
- Leong, K.F.; Cheah, C.M.; Chua, C.K. Solid freeform fabrication of three-dimensional scaffolds for engineering replacement tissues and organs. Biomaterials 2003, 24, 2363–2378.
- Eshraghi, S.; Das, S. Mechanical and microstructural properties of polycaprolactone scaffolds with one-dimensional, two-dimensional, and three-dimensional orthogonally oriented porous architectures produced by selective laser sintering. Acta Biomater. 2010, 6, 2467–2476.
- Miranda, P.; Pajares, A.; Guiberteau, F. Finite element modeling as a tool for predicting the fracture behavior of robocast scaffolds. Acta Biomater. 2008, 4, 1715–1724.
- Miranda, P.; Saiz, E.; Gryn, K.; Tomsia, A.P. Sintering and robocasting of β-tricalcium phosphate scaffolds for orthopaedic applications. Acta Biomater. 2006, 2, 457–466.
- Zhou, K.; Dong, C.; Zhang, X.; Shi, L.; Chen, Z.; Xu, Y.; Cai, H. Preparation and characterization of nanosilver-doped porous hydroxyapatite scaffolds. Ceram. Int. 2015, 41, 1671–1676.
- Rodriguez, G.; Dias, J.; D’Ávila, M.A.; Bártolo, P. Influence of Hydroxyapatite on Extruded 3D Scaffolds. Procedia Eng. 2013, 59, 263–269.
- Shuai, C.; Mao, Z.; Lu, H.; Nie, Y.; Hu, H.; Peng, S. Fabrication of porous polyvinyl alcohol scaffold for bone tissue engineering via selective laser sintering. Biofabrication 2013, 5, 015014.
- Wieding, J.; Jonitz, A.; Bader, R. The Effect of Structural Design on Mechanical Properties and Cellular Response of Additive Manufactured Titanium Scaffolds. Materials 2012, 5, 1336–1347.
- Serra, T.; Planell, J.; Navarro, M. High-resolution PLA-based composite scaffolds via 3-D printing technology. Acta Biomater. 2013, 9, 5521–5530.
- Cox, S.C.; Thornby, J.A.; Gibbons, G.J.; Williams, M.A.; Mallick, K.K. 3D printing of porous hydroxyapatite scaffolds intended for use in bone tissue engineering applications. Mater. Sci. Eng. C 2015, 47, 237–247.
- Inzana, J.A.; Olvera, D.; Fuller, S.M.; Kelly, J.P.; Graeve, O.A.; Schwarz, E.M.; Kates, S.L.; Awad, H.A. 3D printing of composite calcium phosphate and collagen scaffolds for bone regeneration. Biomaterials 2014, 35, 4026–4034.
- Elomaa, L.; Teixeira, S.; Hakala, R.; Korhonen, H.; Grijpma, D.W.; Seppälä, J.V. Preparation of poly(ε-caprolactone)-based tissue engineering scaffolds by stereolithography. Acta Biomater. 2011, 7, 3850–3856.
- Gauvin, R.; Chen, Y.-C.; Lee, J.W.; Soman, P.; Zorlutuna, P.; Nichol, J.W.; Bae, H.; Chen, S.; Khademhosseini, A. Microfabrication of complex porous tissue engineering scaffolds using 3D projection stereolithography. Biomaterials 2012, 33, 3824–3834.
- Lowther, M.; Louth, S.; Davey, A.; Hussain, A.; Ginestra, P.; Carter, L.; Eisenstein, N.; Grover, L.; Cox, S. Clinical, industrial, and research perspectives on powder bed fusion additively manufactured metal implants. Addit. Manuf. 2019, 28, 565–584.
- Zein, I.; Hutmacher, D.W.; Tan, K.C.; Teoh, S.H. Fused deposition modeling of novel scaffold architectures for tissue engineering applications. Biomaterials 2002, 23, 1169–1185.
- Ozbolat, I.T.; Hospodiuk, M. Current advances and future perspectives in extrusion-based bioprinting. Biomaterials 2016, 76, 321–343.
- Li, X.; Liu, B.; Pei, B.; Chen, J.; Zhou, D.; Peng, J.; Zhang, X.; Jia, W.; Xu, T. Inkjet Bioprinting of Biomaterials. Chem. Rev. 2020, 120, 10793–10833.
- Ng, W.L.; Lee, J.M.; Yeong, W.Y.; Naing, M.W. Microvalve-based bioprinting—Process, bio-inks and applications. Biomater. Sci. 2017, 5, 632–647.
- Ng, W.L.; Lee, J.M.; Zhou, M.; Chen, Y.-W.; Lee, K.-X.A.; Yeong, W.Y.; Shen, Y.-F. Vat polymerization-based bioprinting—process, materials, applications and regulatory challenges. Biofabrication 2019, 12, 022001.
- Zhuang, P.; Ng, W.L.; An, J.; Chua, C.K.; Tan, L.P. Layer-by-layer ultraviolet assisted extrusion-based (UAE) bioprinting of hydrogel constructs with high aspect ratio for soft tissue engineering applications. PLoS ONE 2019, 14, e0216776.
- Li, Q.; Lei, X.; Wang, X.; Cai, Z.; Lyu, P.; Zhang, G. Hydroxyapatite/Collagen Three-Dimensional Printed Scaffolds and Their Osteogenic Effects on Human Bone Marrow-Derived Mesenchymal Stem Cells. Tissue Eng. Part A 2019, 25, 1261–1271.
- Cheah, C.; Chua, C.; Leong, K.F.; Chua, S.W. Development of a Tissue Engineering Scaffold Structure Library for Rapid Prototyping. Part 1: Investigation and Classification. Int. J. Adv. Manuf. Technol. 2003, 21, 291–301.
- Wettergreen, M.; Bucklen, B.; Starly, B.; Yuksel, E.; Sun, W.; Liebschner, M. Creation of a unit block library of architectures for use in assembled scaffold engineering. Comput. Des. 2005, 37, 1141–1149.
- Hutmacher, D.W. Scaffolds in tissue engineering bone and cartilage. Biomaterials 2000, 21, 2529–2543.
- Brown, B.N.; Valentin, J.E.; Stewart-Akers, A.M.; McCabe, G.P.; Badylak, S.F. Macrophage phenotype and remodeling outcomes in response to biologic scaffolds with and without a cellular component. Biomaterials 2009, 30, 1482–1491.
- Yannas, I.V.; Lee, E.; Orgill, D.P.; Skrabut, E.M.; Murphy, G.F. Synthesis and characterization of a model extracellular matrix that induces partial regeneration of adult mammalian skin. Proc. Natl. Acad. Sci. USA 1989, 86, 933–937.
- Murphy, C.M.; O’Brien, F.J. Understanding the effect of mean pore size on cell activity in collagen-glycosaminoglycan scaffolds. Cell Adhes. Migr. 2010, 4, 377–381.
- Martin, Y.; Vermette, P. Bioreactors for tissue mass culture: Design, characterization, and recent advances. Biomaterials 2005, 26, 7481–7503.
- Raimondi, M.T.; Moretti, M.; Cioffi, M.; Giordano, C.; Boschetti, F.; Laganà, K.; Pietrabissa, R. The effect of hydrodynamic shear on 3D engineered chondrocyte systems subject to direct perfusion. Biorheology 2006, 43, 215–222.
- Boschetti, F.; Raimondi, M.T.; Migliavacca, F.; Dubini, G. Prediction of the micro-fluid dynamic environment imposed to three-dimensional engineered cell systems in bioreactors. J. Biomech. 2006, 39, 418–425.
- Naing, M.; Chua, C.; Leong, K.F.; Wang, Y. Fabrication of customised scaffolds using computer-aided design and rapid prototyping techniques. Rapid Prototyp. J. 2005, 11, 249–259.
- Wubneh, A.; Tsekoura, E.K.; Ayranci, C.; Uludağ, H. Current state of fabrication technologies and materials for bone tissue engineering. Acta Biomater. 2018, 80, 1–30.
- Barba, A.; Maazouz, Y.; Diez-Escudero, A.; Rappe, K.; Espanol, M.; Montufar, E.B.; Öhman-Mägi, C.; Persson, C.; Fontecha, P.; Manzanares, M.-C.; et al. Osteogenesis by foamed and 3D-printed nanostructured calcium phosphate scaffolds: Effect of pore architecture. Acta Biomater. 2018, 79, 135–147.
- Kantaros, A.; Chatzidai, N.; Karalekas, D. 3D printing-assisted design of scaffold structures. Int. J. Adv. Manuf. Technol. 2016, 82, 559–571.
- Habib, F.N.; Iovenitti, P.; Masood, S.H.; Nikzad, M. Fabrication of polymeric lattice structures for optimum energy absorption using Multi Jet Fusion technology. Mater. Des. 2018, 155, 86–98.
- Hollister, S.J. Scaffold Design and Manufacturing: From Concept to Clinic. Adv. Mater. 2009, 21, 3330–3342.
- Krishna, L.; Kamal, M.; Venkatesh, S. Design and manufacturing of a scaffold for biomedical applications using additive manufacturing. Indian J. Sci. Res. 2017, 15, 1–6.
- Ning, L.; Chen, X. A brief review of extrusion-based tissue scaffold bio-printing. Biotechnol. J. 2017, 8.
- Nurulhuda, N. Fabrication PEGDA/ANFs Biomaterial as 3D Tissue Engineering Scaffold by DLP 3D Printing Tecshnology. Int. J. Eng. Adv. Technol. 2019, 8, 751–758.
- Taylor, S.L.; Ibeh, A.J.; Jakus, A.E.; Shah, R.N.; Dunand, D.C. NiTi-Nb micro-trusses fabricated via extrusion-based 3D-printing of powders and transient-liquid-phase sintering. Acta Biomater. 2018, 76, 359–370.
- Yang, Y.; Chu, L.; Yang, S.; Zhang, H.; Qin, L.; Guillaume, O.; Eglin, D.; Richards, R.G.; Tang, T. Dual-functional 3D-printed composite scaffold for inhibiting bacterial infection and promoting bone regeneration in infected bone defect models. Acta Biomater. 2018, 79, 265–275.
- Hu, Y.; Wang, J.; Li, X.; Hu, X.; Zhou, W.; Dong, X.; Wang, C.; Yang, Z.; Binks, B.P. Facile preparation of bioactive nanoparticle/poly(ε-caprolactone) hierarchical porous scaffolds via 3D printing of high internal phase Pickering emulsions. J. Colloid Interface Sci. 2019, 545, 104–115.
- Alison, L.; Menasce, S.; Bouville, F.; Tervoort, E.; Mattich, I.; Ofner, A.; Studart, A.R. 3D printing of sacrificial templates into hierarchical porous materials. Sci. Rep. 2019, 9, 1–9.
- Minas, C.; Carnelli, D.; Tervoort, E.; Studart, A.R. 3D Printing of Emulsions and Foams into Hierarchical Porous Ceramics. Adv. Mater. 2016, 28, 9993–9999.
- Lefevere, J.; Protasova, L.; Mullens, S.; Meynen, V. 3D-printing of hierarchical porous ZSM-5: The importance of the binder system. Mater. Des. 2017, 134, 331–341.
- Ng, W.L.; Goh, M.H.; Yeong, W.Y.; Naing, M.W. Applying macromolecular crowding to 3D bioprinting: Fabrication of 3D hierarchical porous collagen-based hydrogel constructs. Biomater. Sci. 2018, 6, 562–574.
- Chen, Y.W.; Shen, Y.F.; Ho, C.C.; Yu, J.; Wu, Y.H.A.; Wang, K.; Shih, C.T.; Shie, M.Y. Osteogenic and angiogenic potentials of the cell-laden hydrogel/mussel-inspired calcium silicate complex hierarchical porous scaffold fabricated by 3D bioprinting. Mater. Sci. Eng. C 2018, 91, 679–687.
- Kovalcik, A.; Sangroniz, L.; Kalina, M.; Skopalova, K.; Humpolíček, P.; Omastova, M.; Mundigler, N.; Müller, A.J. Properties of scaffolds prepared by fused deposition modeling of poly(hydroxyalkanoates). Int. J. Biol. Macromol. 2020, 161, 364–376.
- Odde, D.J.; Renn, M.J. Laser-guided direct writing of living cells. Biotechnol. Bioeng. 2000, 67, 312–318.
- Pardo, L.; Wilson, J.W.C.; Boland, T. Characterization of Patterned Self-Assembled Monolayers and Protein Arrays Generated by the Ink-Jet Method. Langmuir 2003, 19, 1462–1466.
