Bisindoles are structurally complex dimers and are intriguing targets for partial and total synthesis. They exhibit stronger biological activity than their corresponding monomeric units. Bisindole alkaloids are naturally occurring alkaloids containing two indole nuclei and are the products of late-stage biosynthetic processes in higher plants by combining two monomeric units. Depending on the monomeric units involved, bisindoles can be a homo- or heterodimer. As a result, bisindole alkaloids comprise much higher structural complexity than both of the monomeric units that comprise them.
Alstonia
, a major genus in the Apocynaceae family of plants, has more than 150 species and is found all over the world. Robert Brown named it in 1811 in honor of Charles Alston (1685–1760), an eminent botanist at the University of Edinburgh. The
Alstonia
genus’ trees and shrubs are prevalent in the tropical and subtropical parts of Africa, Asia, and Australia. They contribute significant pharmacological activity, including anticancer, antileishmanial, antimalarial, antitussive, antiviral, antiarthritic, and antibacterial activities.
- Apocynaceae
- Alstonia species
- Sarpagine-macroline-ajmaline type indole alkaloids
- Pleiocarpamine
- Bisindole synthesis
- Isolation of bisindoles
- Bioactivity of bisindoles
1. Bisindole Alkaloids in drug discovery
Nature has been a substantial and sustainable pool of biologically active compounds. Since ancient times natural product extracts (in crude form) have been used in traditional and folk medicines in many countries. In modern times pure (isolated) natural products and their derivatives play an important role in drug discovery, as indicated by their prevalence in approved drugs for clinical use. Out of the 1881 newly FDA-approved drugs over the last four decades (1 January 1981 to 30 September 2019), a significant portion comprising 506 (26.9%) were either natural products or derived from or inspired by natural products [1]. It is expected that the advent of modern and innovative technologies such as computational software, cheminformatics, artificial intelligence, automation, and quantum computing will further boost natural product-based drug discovery. A synergy among these technological milestones would accelerate hit to lead to clinic pathways of drug discovery, and natural products are expected to remain an important source [2]. Moreover, pharmacophores and their unique stereochemical interactions with natural products may stimulate more demanding targets such as protein–protein interactions in the near future and open up a new avenue in modern drug discovery [3]. The majority of biologically active natural products are produced in plants, known traditionally as medicinal plants. Alkaloids, the most important class of natural products with structural diversity and significant pharmacological effects, are mainly found in higher plants such as the Apocynaceae, Ranunculaceae, Papaveraceae, and Leguminosae families [4][11]. These natural products, along with flavonoids, fatty acids, etc., are the major classes of secondary metabolites that are believed to be parts of the plants’ defense mechanism. To date, many monoterpenoid indole and bisindole alkaloids have been found in the Alstonia genus [5][4]. Modern clinical application of many of these alkaloids are similar to their traditional or folklore applications; for example, cocaine and morphine were used as anesthetics while caffeine and nicotine were used as stimulants [6][12]. Recently, Fielding et al. illustrated that several anti-coronavirus alkaloids showed potential therapeutic value against severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) in their in silico studies [7][9].
2. Isolation and Plant' Morphology of Bisindoles from Alstonia Species
Among various species of the
Alstonia
A. macrophylla
A. angustifolia
vide infra
1
https://doi.org/10.3390/molecules26113459
of A. macrophylla [12] and the leaves, stem-bark, and root-bark extracts of
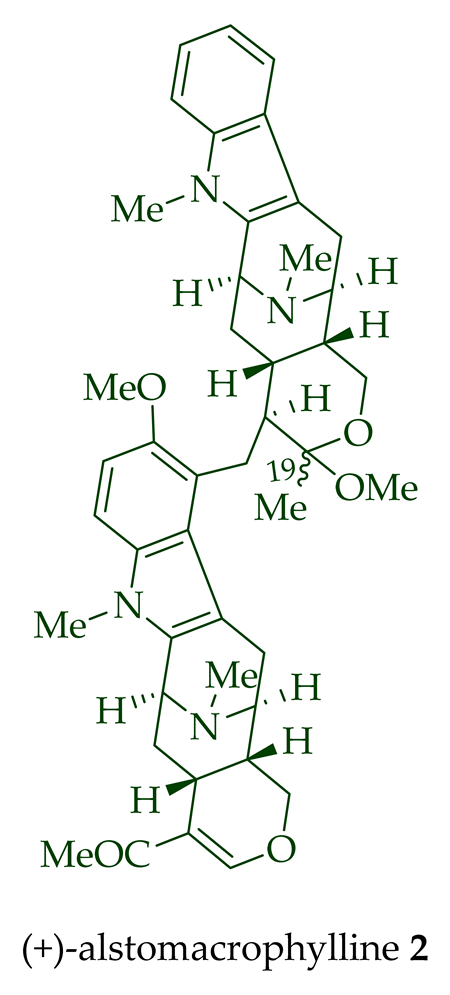
A. scholaris, A. glaucescens, and A. macrophylla[13][14]. (+)-Alstomacrophylline
2
of A. macrophylla [12] and the leaves, stem-bark, and root-bark extracts of
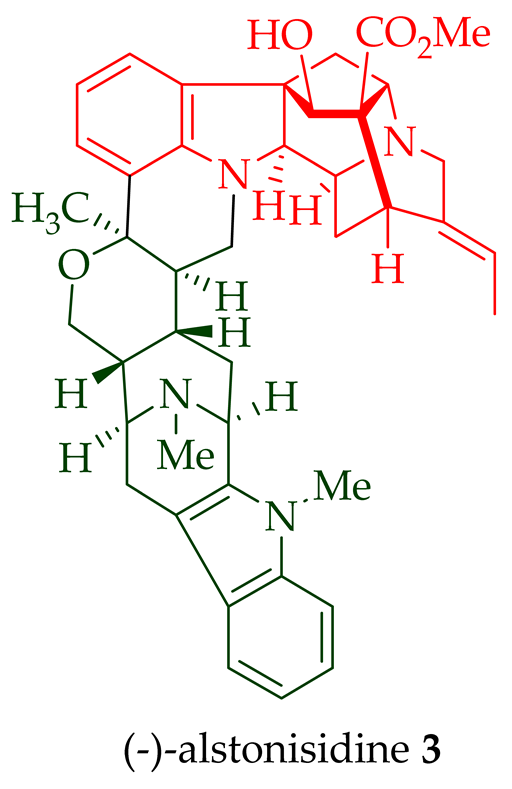
A. scholaris, A. glaucescens, and A. macrophylla [14]. (-) Alstonisidine
3
A. muelleriana [15][16]. The structure of (-)-alstonisidine
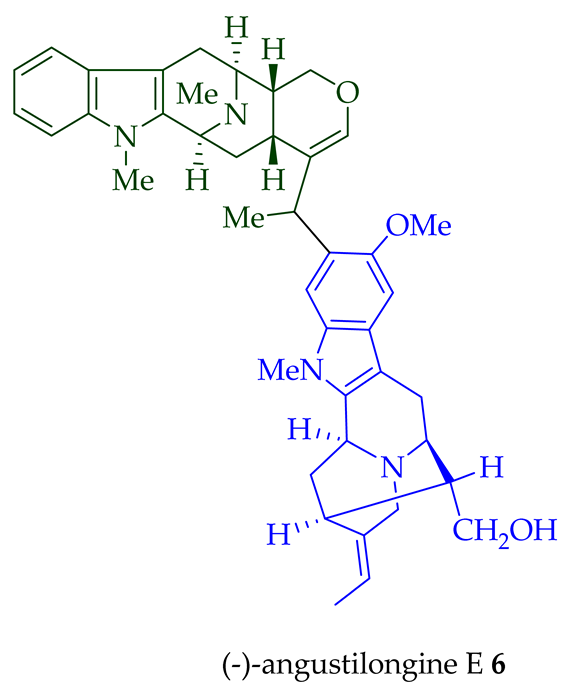
3 was confirmed by X-ray crystallographic data [17]. Yeap et al. recently isolated seven novel bisindoles from the methanol extract of the stem-bark of Malayan
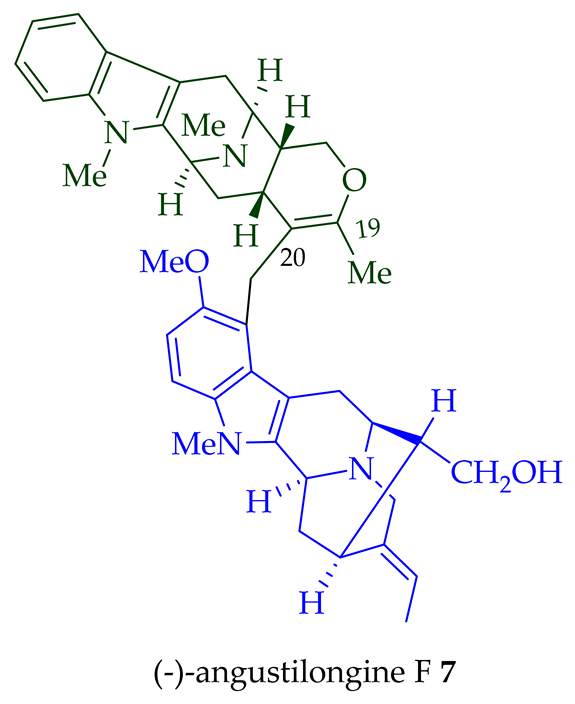
A. penangiana [18]. This includes (-)-angustilongine E
6
7
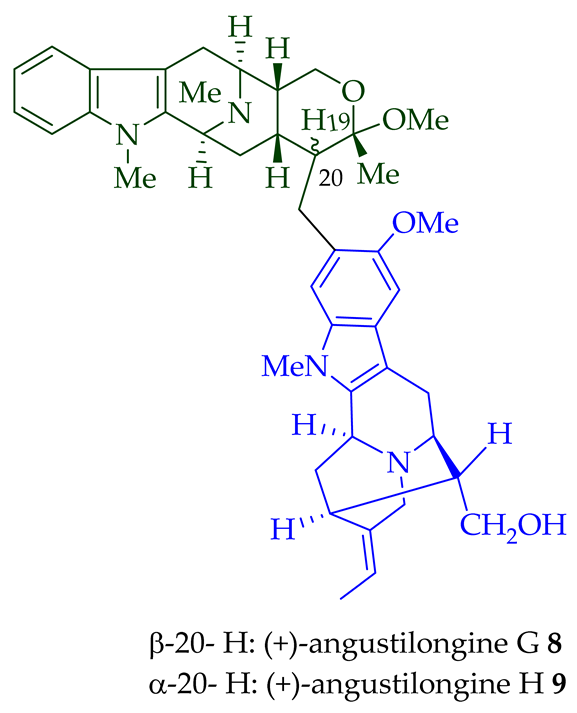
8
9
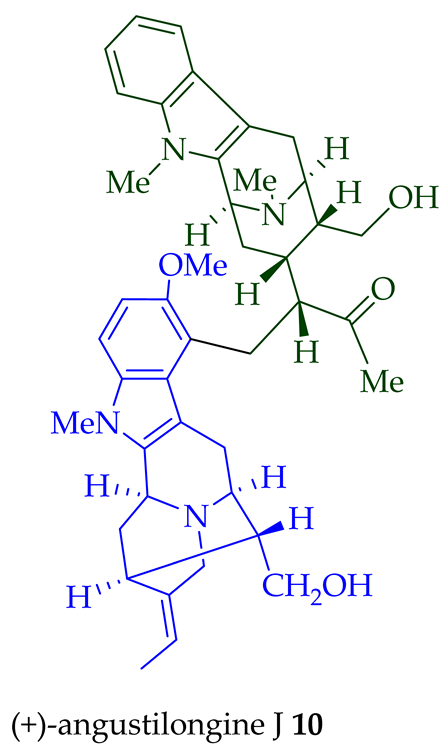
10
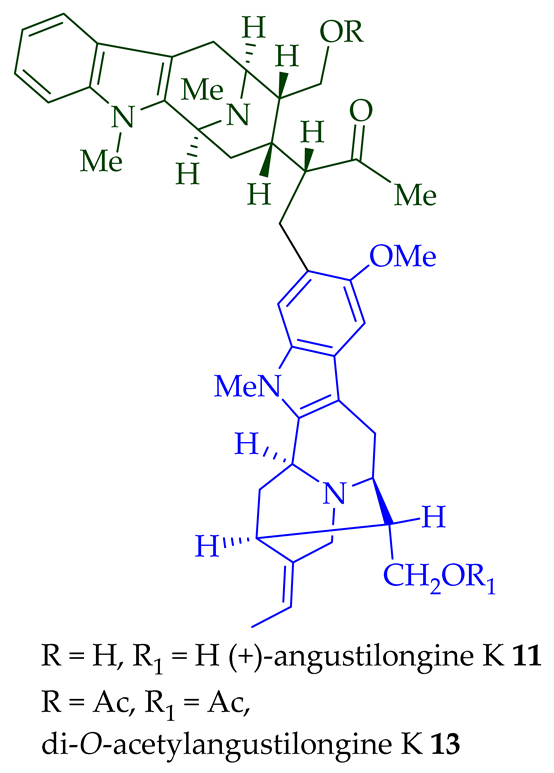
11
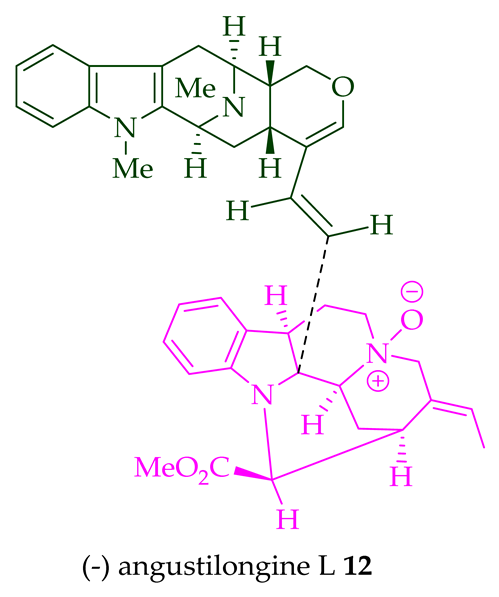
12
11
di
O
13
95% yield [18]. Among those, angustilongine G
8
9 are C-19 methyl substituted [19] bisindoles. The structures of the angustilongines were confirmed by various spectroscopic data, including
1
13C NMR, 2D NMR, IR, and HRMS by Yeap et al. [18]. Angustilongine E
6
7
8
9
10
11
8
9 differ in stereochemistry only at the C-20 position.
Table 1.
Alstonia
| Macroline-macroline type | ||
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
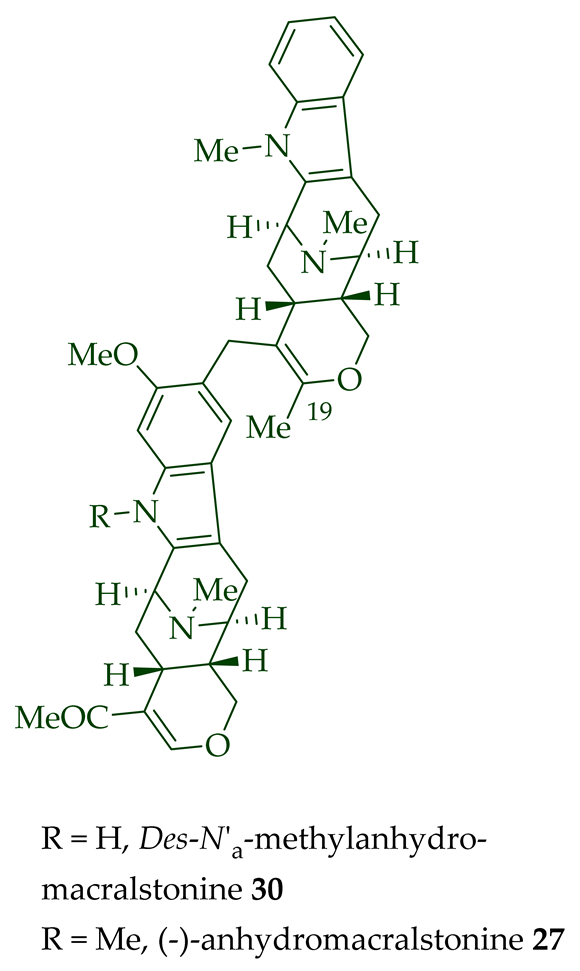
[14]. (-)-Anhydromacralstonine
27
A. angustiloba [23] and contains (-)-alstophylline and (+)-macroline as monomeric units. Another (-)-alstophylline
28
Des-N’a
30,
A. muelleriana [14][30], the stem-bark of
A. angustifolia [28], and
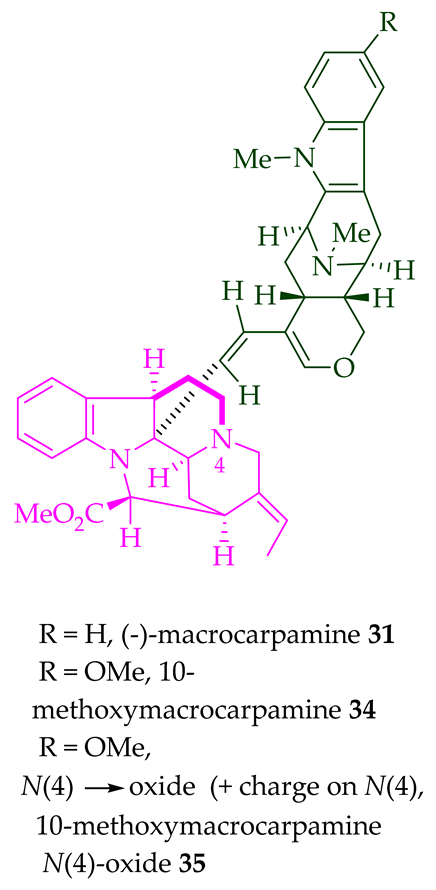
A. glabriflora [26]. (+)-Macrocarpamine
31
A. scholaris, A. glaucescens, and A. macrophylla [14], as well as the stem-bark of
A. angustifolia [23]. 10-Methoxymacrocarpamine
34
N4′
35
A. angustifolia leaves [31].
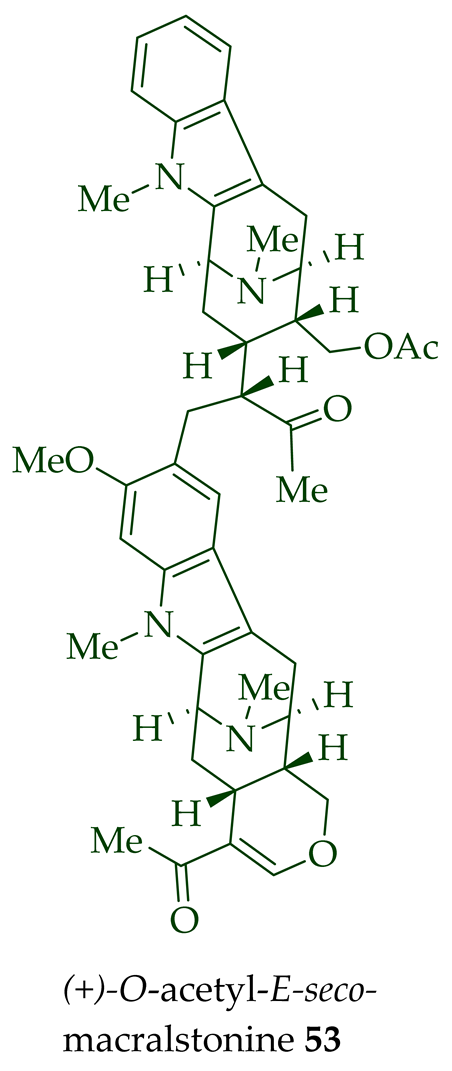
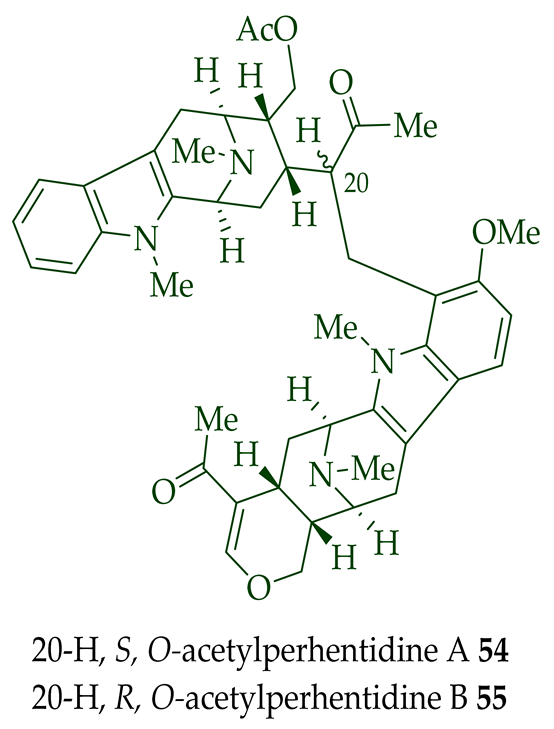
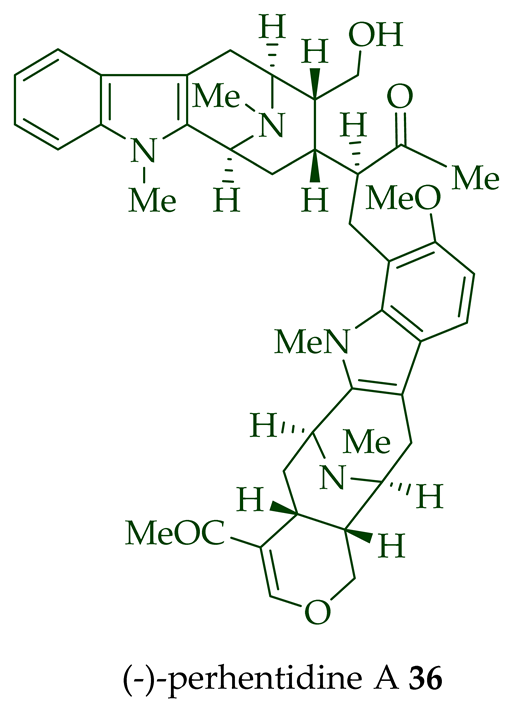
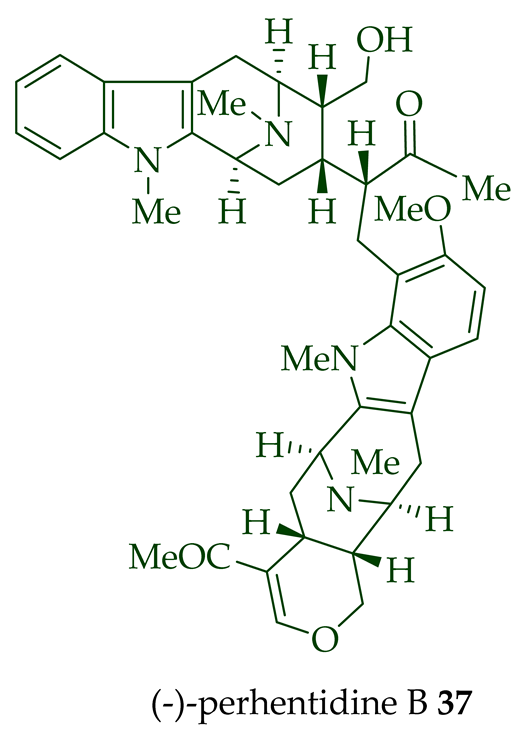
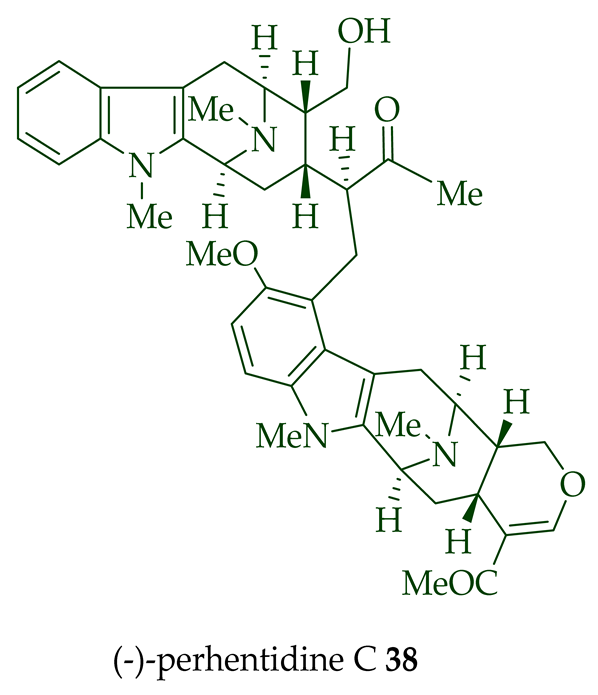
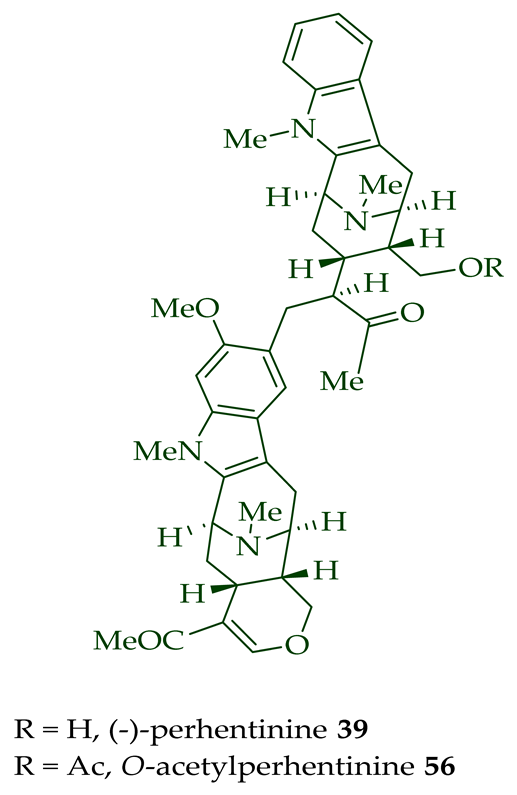
Lim et al. [28] reported the isolation of new macroline–macroline-type bisindoles, (-)-perhentidine A
36
37
38
A. macrophylla
A. angustifolia.
39
A. macrophylla
A. angustifolia [12]. The exact structure of (-)-perhentinine
39
39
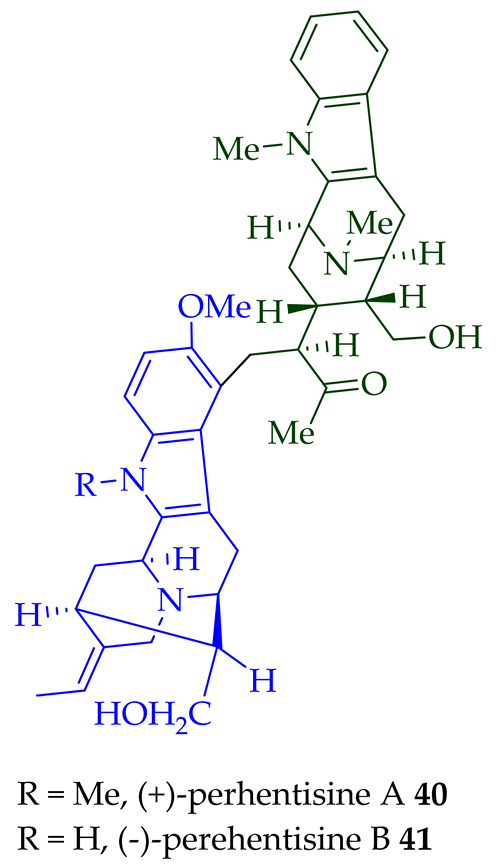
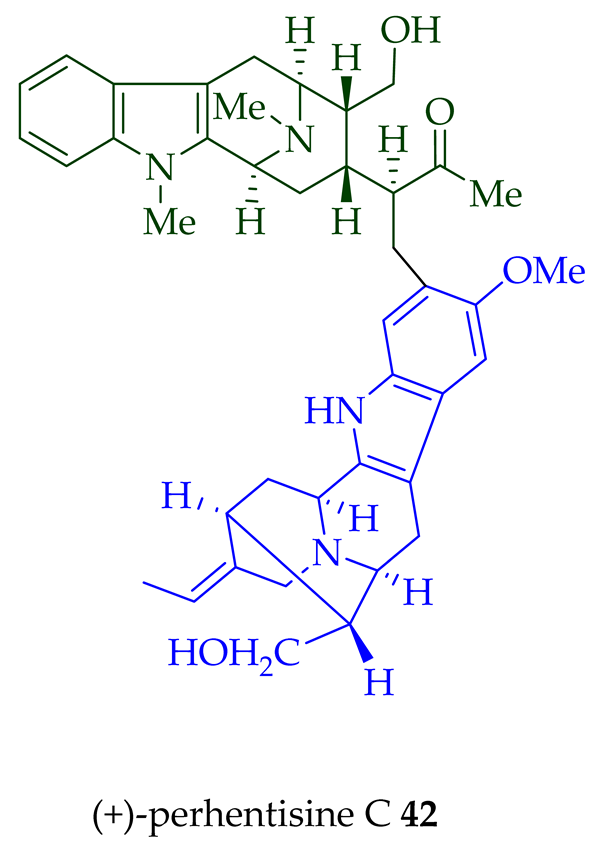
36–38) [28]. Tan et al. isolated three macroline–sarpagine-type bisindoles: (+)-perhentisine A
40
41
42
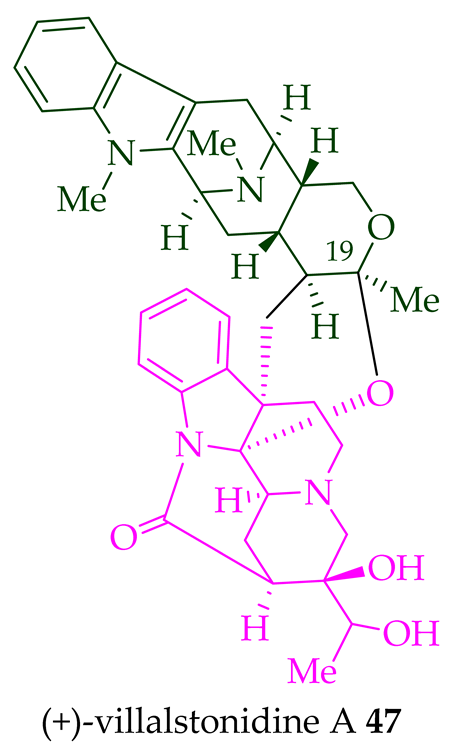
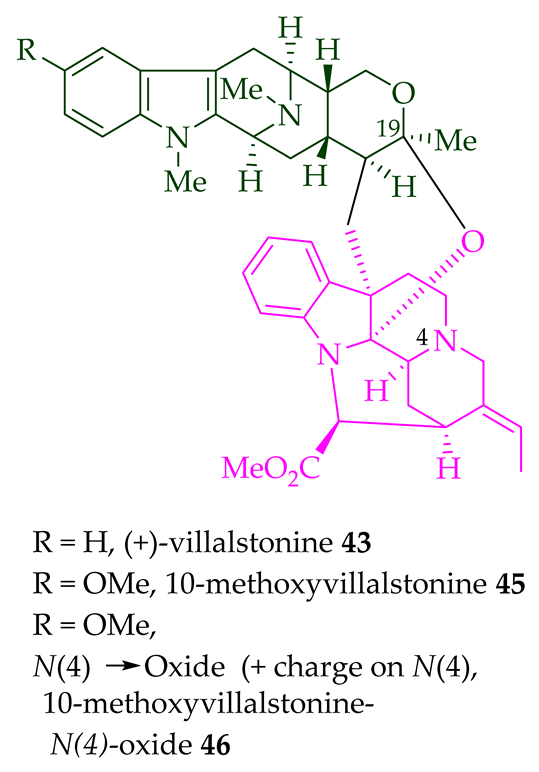
A. angustifolia as a light yellow-colored oil together with other bisindoles [23]. The structures of these bisindoles were also elucidated using various NMR and MS techniques [23]. (+)-Villastonine
43
Alstonia
A. spectabilis [24] and
A. muelleriana [32] by LeQuesne et al.,
A. macrophylla [25][27][33], and
A. angustifolia [31][34]. Schmid et al. elucidated the structure of (+)-villalstonine
43 by spectroscopic means, accompanied by degradation, and Nordman et al. confirmed the structure by X-ray crystallography [33][35].
Table 2.
Alstonia
| Bisindoles | Alstonia Species |
Morphology and References |
|---|---|---|
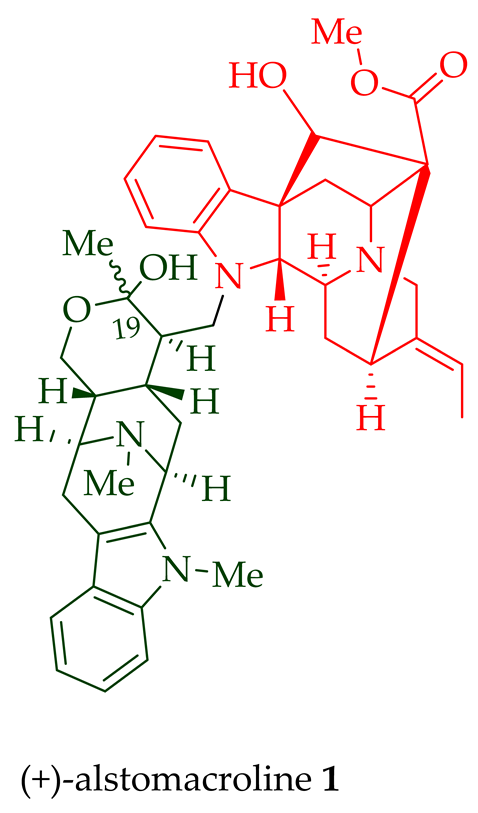
| (+)-Alstomacroline 1 | A. scholaris, A. glaucescens, and A. macrophylla extracts | Leaves, stem-bark, and root-bark [13][14][19,20] |
| A. macrophylla | Bark [12][18] | |
| (+)-Alstomacrophylline 2 | A. macrophylla | Bark [13][19] |
| A. scholaris, A. glaucescens, and A. macrophylla extracts | Leaves, stem-bark, and root-bark [14][20] | |
 |
 |
 |
| Macroline-sarpagine type | ||
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
| Macroline-ajmaline type | ||
 |
 |
|
| Macroline-pleiocarpamine type | ||
 |
 |
 |
 |
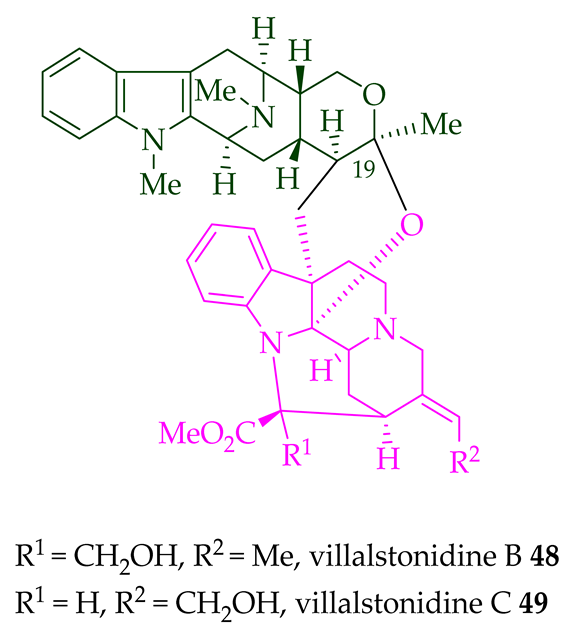 |
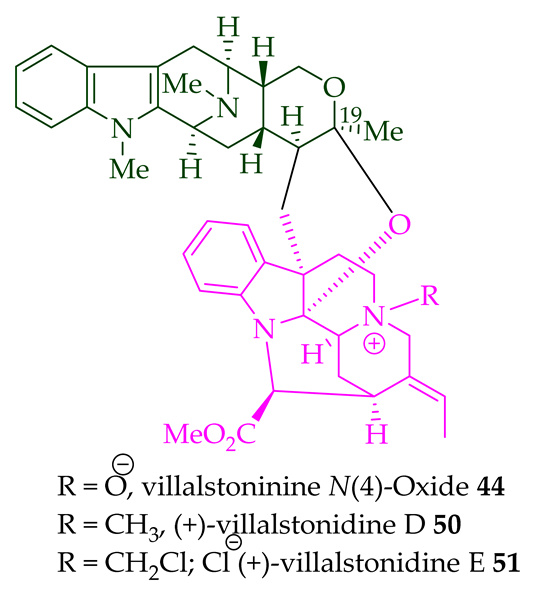 |
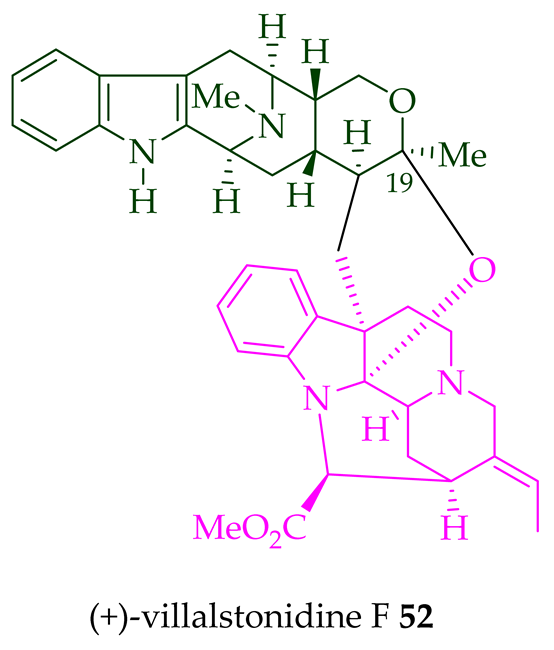 |
||
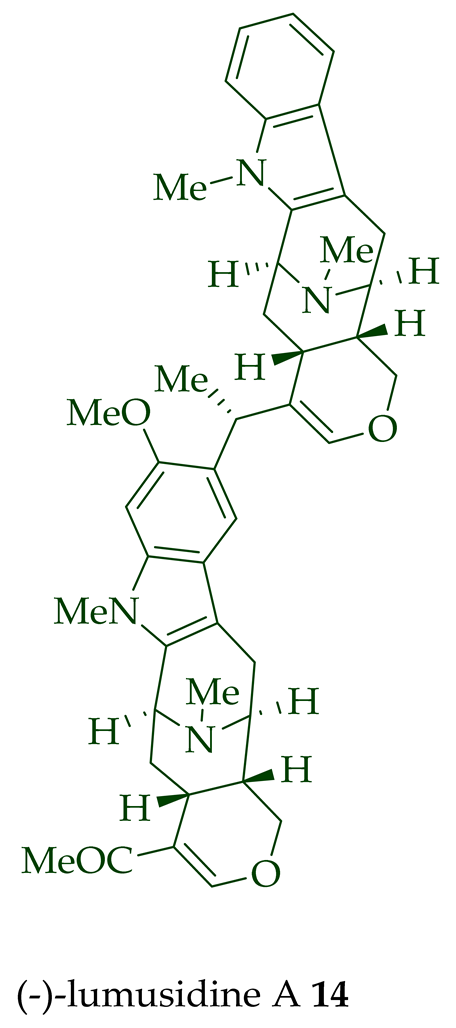
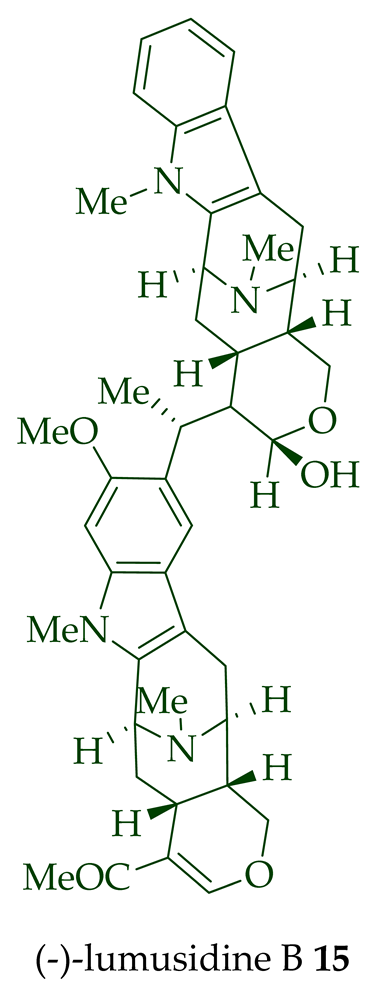
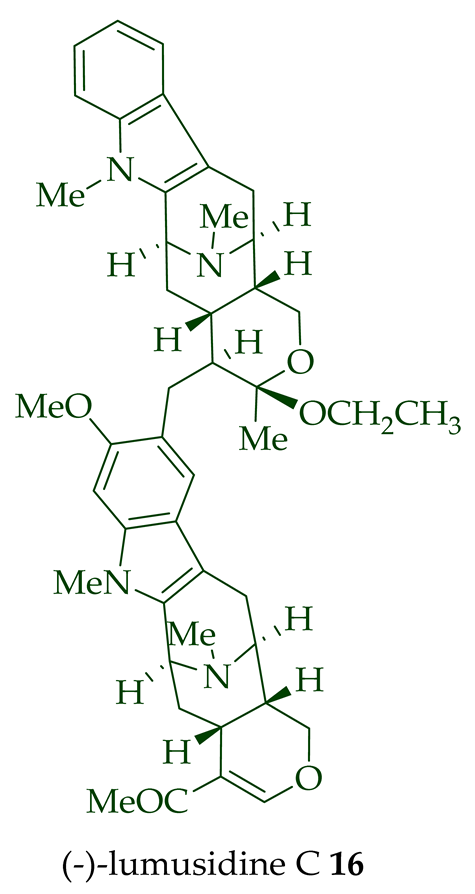
14
15
16
17
A. macrophylla and the structures were confirmed via NMR spectroscopy, mass spectrometry, UV spectroscopy, and X-ray crystallography [12]. After isolation, the group of Kam et al. converted oily (-)-lumusidine A
14
15
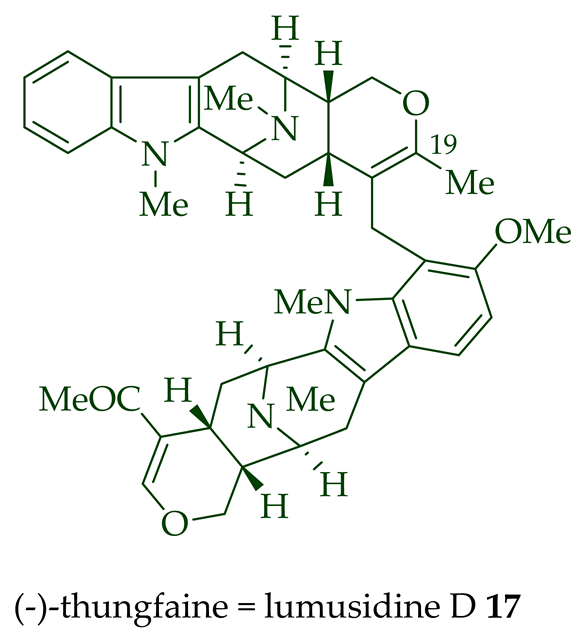
17 into the corresponding crystalline dimethyl diiodide salts (structures not shown) by treatment with an excess of iodomethane for 24 h. The crystalline salts were employed to obtain X-ray crystallographic data to elucidate the exact stereochemical confirmation [12]. (-)-Lumusidine D
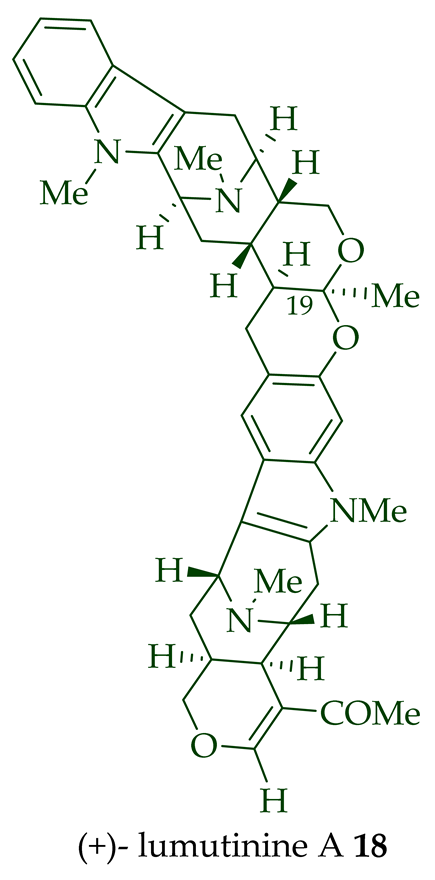
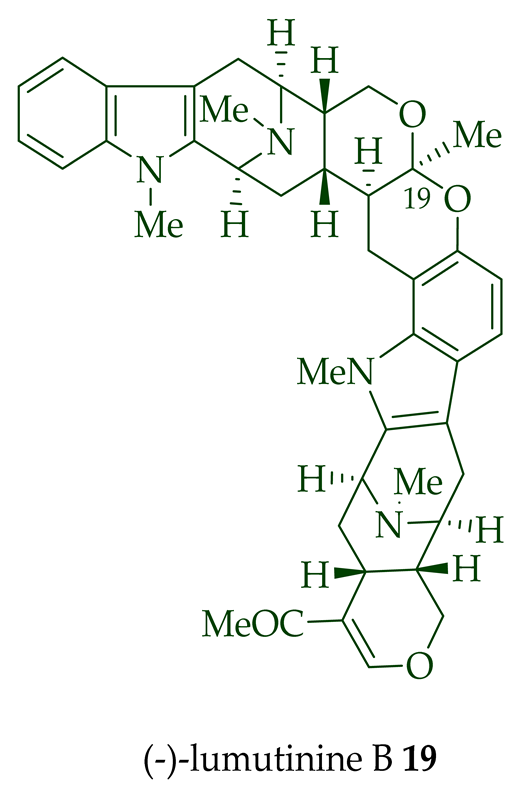
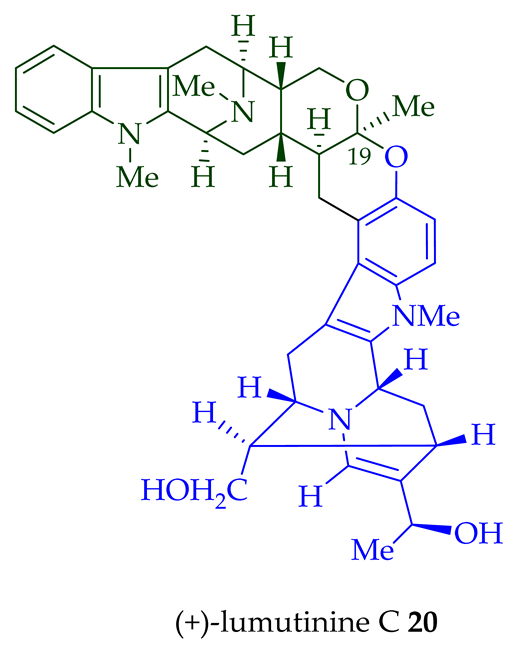
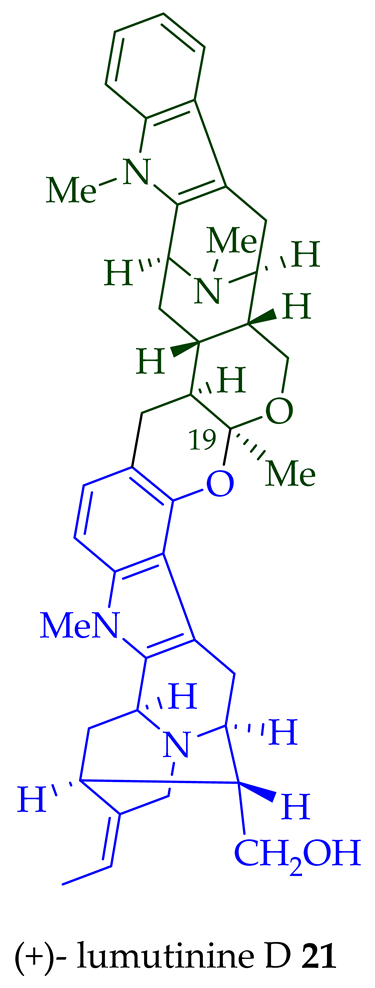
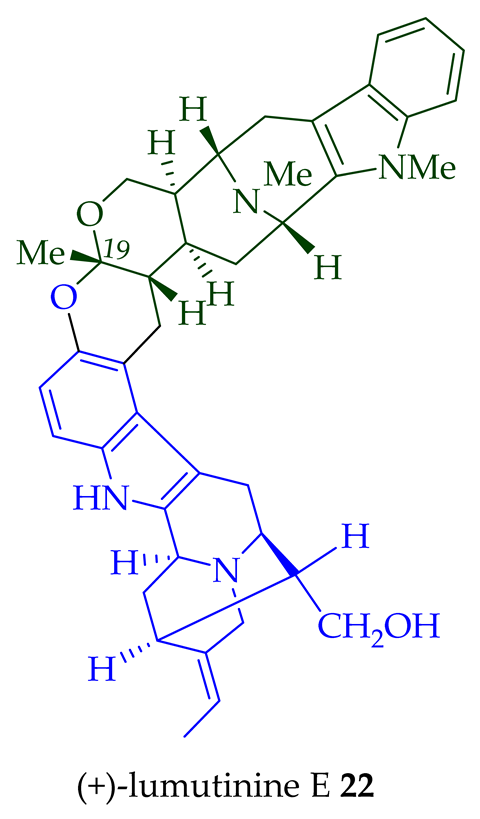
17 is also known as thungfaine [20]. (+)-Lumutinine A
18
19
20
21
A. macrophylla as a light yellowish oil [21]. (+)-Lumutinine A
18
19
20
21
22 are macroline–sarpagine-type bisindoles. The structures of the lumutinines were elucidated using spectroscopic means including 1D and 2D NMR, IR, as well as mass spectrometric analysis [21]. The structure of (+)-lumutinine D
21 was confirmed by X-ray crystallographic data [22]. (+)-Lumutinine E
22
A. angustifolia [23].
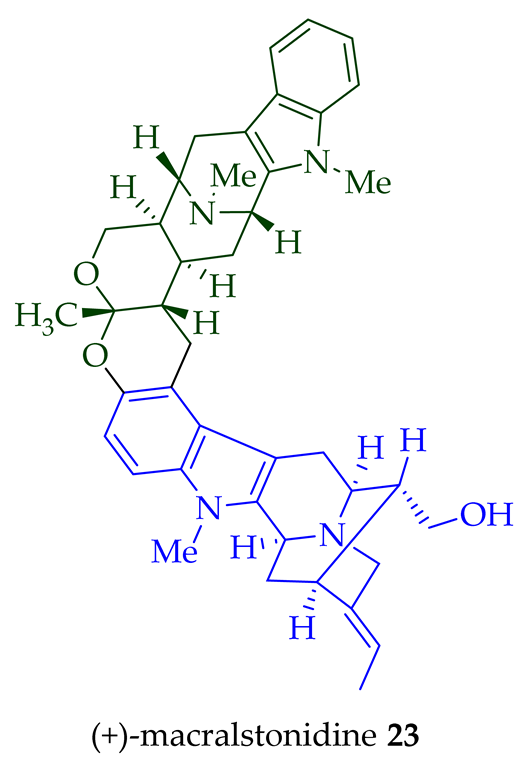
23
A. macrophylla [24][25], as well as from
A. somersentenis [24] and
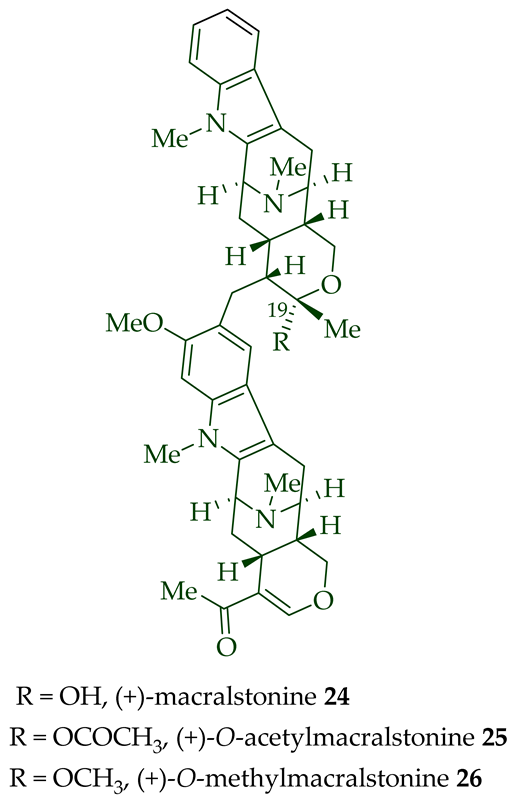
A. spectabilis [26]. (+)-Macralstonine
24
A. scholaris, A. glaucescens, and A. macrophylla extracts [14],
A. macrophylla [24][25][27][28][29],
A. muelleriana [30],
A. angustifolia [31], as well as from
A. glabriflora [26]. The structure of (+)-macralstonine
24 was confirmed by various NMR spectroscopy, mass spectrometry, and X-ray crystallography [28]. The (+)-macralstonine
24
O
25,
