Electrospun nanofibers had been gaining importance in several areas such as the biomedical, environmental, food, textile, and biotechnology industries, amongst others. The fabrication of three-dimensional membranes through the electrospinning technique confers several characteristics that are important in the above industrial approaches such as high-to-volume radio, high surface area, controllable porosity, thickness, and mechanical properties, also the non-toxicity, biocompatibility, and biodegradability can be conferred to the scaffolds by choosing the adequate polymeric formulation. This entry discusses the characteristics and importance of electrospun nanofibers in industry.
- Parameters
- Polymers
- Tissue engineering
- Biomaterials
- Drug delivery systems
- Electrospun nanofibers
- Electrospinning
1. Electrospinning Ttechnique
1.1. Parameters and conditions of electrospinning
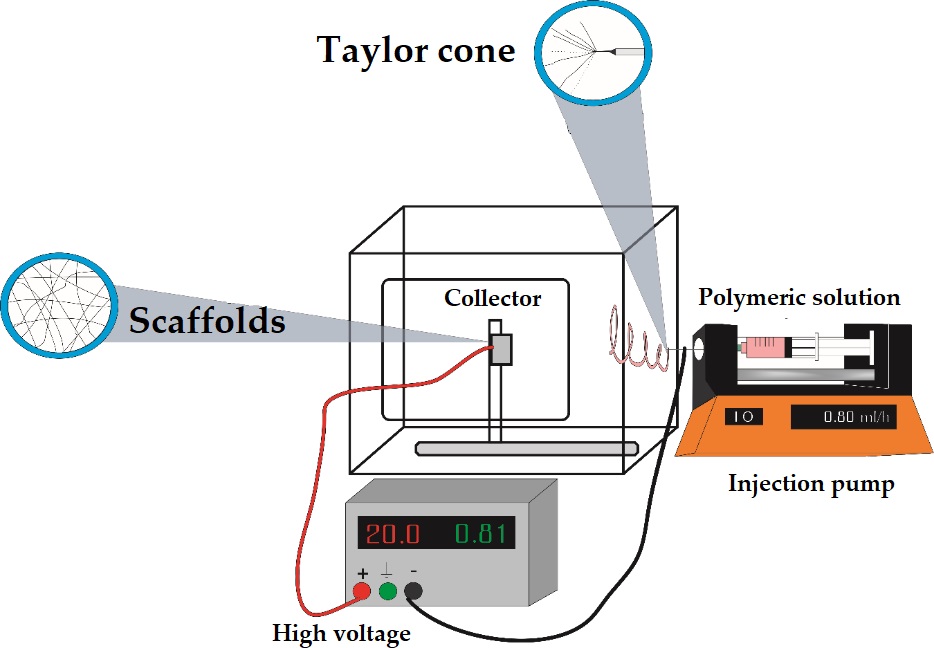
The molecular weight of the chosen polymer, the concentration used of the polymer solution, and the final viscosity are the main parameters that determine fiber formation[4]. Moreover, the final characteristic of the fibers comes from the intrinsic properties of the base polymer. This polymer solution is then loaded to a normal syringe which is mounted in a syringe pump that injects the polymeric solution to a certain rate (volume/time). The injected solution creates a drop that is charged to a high potential between 15 and 50 kV. When the voltage is applied, free electrons, ions, or ion pairs are produced and are attracted to the formed electrical field. This stimulation is adequate for conductive solutions. However, for non-conducting solutions, the voltage may be directly applied to the fluids by incorporating salts[14].
1.1.2. Fiber formation
The polarization of the polymeric solutions depends upon the applied voltage, generating the propulsion of a polymeric jet, this voltage works to break the surface tension and promote the fluid elasticity in the polymer solution to distort the droplet into a conical-shaped structure known as the Taylor cone[15].
The formed jet falls to the ground creating a slight continuous polymeric filament. Then, before reaching the ground, the charged fluid is speeded in the presence of the electrostatic field[16].
The falling fluid is charged and accelerated, and thus stretched and succumb to one or more fluid instabilities which distort, then many spirals and distorted paths are formed, before being collected at the collector electrode. This region of variability is also known as the whipping region[17]
Diverse kinds of collectors can be used, including static collector, rotating drum collectors, rotating wheel with a beveled edge, moving belt collector, multifilament thread, parallel bars, and simple mesh collector[3]
A variety of parameters can be optimized in order to generate nanofibers with controlled morphology, such as:
1.2. Solution-related parameters
Polymer molecular weight: Higher molecular weight, less probability of droplet filling the collector
Polymer concentration: Higher concentration, less dripping, and increased fiber diameter
Solution conductivity: Increasing conductivity reduces defects in the fiber and reduces its diameter
Surface tension: Lower tension favors fiber production
1.3. Processing parameters
Applied voltage: Higher voltage, higher probability of dripping
Distance to collector: Greater distance, smaller diameter in the fibers, and less humidity in them
Flow rate: Higher speed, higher fiber moisture
Collectors: Different collectors, different fiber arrangement
1.4. Environmental parameters
Temperature: Higher temperature, smaller diameter in the fiber
Humidity: Low humidity, fast solvent evaporation[18]
Respecting the fiber morphology, among the electrospinning parameters that affect the fiber morphology, the low concentrations/viscosities yield defects in the form of beads and junctions; this last effect can be avoided by increasing concentration/viscosity which reduces the defects. Fiber diameters can be increased when the concentration/viscosity is incremented. Moreover, the increase of conductivity helps in the production of uniform bead-free fibers. Finally, higher conductivities yield smaller fibers in general[19].
It has been discussed, that a conclusive relationship established between surface tension and fiber morphology does not exist. Increasing polymer molecular weight reduces the number of beads and droplets. On the other hand, successful spinning occurs in solvents with a high dielectric constant. Lower flow rates yield fibers with smaller diameters, and high flow rates may produce fibers that are not dry upon reaching the collector. At extreme high voltage, beading is observed.
Nevertheless, the correlation between voltage and fiber diameter is ambiguous. A minimum distance is required to obtain dried fibers. At distances, either too close or too far, beading is observed. Nanofibers can be synthesized with a great variety of specific secondary structures using different strategies: nanofiber with core/shell structures, hollow interiors, and porous structures[20].
On the other hand, using the coaxial electrospinning technique, hollow fibers are obtained. Multiple needle tips are employed to increase the throughput. Smoother fibers result from metal collectors; highly porous fiber structures are obtained using porous collectors. Aligned fibers are obtained using a conductive frame, rotating drum, or a wheel-like bobbin collector. Finally, increasing temperatures cause a decrease in the solution’s viscosity, thus resulting in smaller fibers; while increasing humidity results in the appearance of circular pores on the fibers[21].
Acknowledgment
The author thanks Daniella Alejandra Pompa Monroy for drawing figure 1.
References
- Luis Jesús Villarreal-Gómez; Ricardo Vera-Graziano; Maria Raquel Vega-Rios; Jose Luis Pineda-Camacho; Paris Astrid Mier-Maldonado; Horacio Almanza-Reyes; Jose Manuel Cornejo Bravo; In Vivo Biocompatibility of Dental Scaffolds for Tissue Regeneration. Advanced Materials Research 2014, 976, 191-195, 10.4028/www.scientific.net/amr.976.191.
- Jose Cornejo-Bravo; Luis Jesús Villarreal-Gómez; Ricardo Vera-Graziano; Maria Raquel Vega-Rios; Jose Luis Pineda-Camacho; Horacio Almaraz-Reyes; Paris Astrid Mier-Maldonado; Biocompatibility Evaluation of Electrospun Scaffolds of Poly (L-Lactide) with Pure and Grafted Hydroxyapatite. Journal of the Mexican Chemical Society 2017, 58, 435-443, 10.29356/jmcs.v58i4.53.
- Rodolfo Daniel Velasco Barraza, Alan Saúl Álvarez Suarez, Luis Jesús Villarreal Gómez, Juan Antonio Paz González, Ana Leticia Iglesias, Ricardo Vera Graziano; Designing a low cost electrospinning device for practical learning in a bioengineering biomaterials course. Revista Mexicana de Ingeniería Biomédica 2015, 37, 27-36, 10.17488/rmib.37.1.1.
- Luis Jesús Villarreal-Gómez; José Manuel Cornejo-Bravo; Ricardo Vera-Graziano; Daniel Grande; Villarreal-Gómez Luis Jesús; Cornejo-Bravo José Manuel; Vera-Graziano Ricardo; Grande Daniel; Electrospinning as a Powerful Technique for Biomedical Applications: A Critically Selected Survey. Journal of Biomaterials Science, Polymer Edition 2015, 27, 1-32, 10.1080/09205063.2015.1116885.
- Erick José Torres-Martinez; José Manuel Cornejo Bravo; Aracely Serrano Medina; Graciela Lizeth Pérez-González; Luis Jesús Villarreal Gómez; A Summary of Electrospun Nanofibers as Drug Delivery System: Drugs Loaded and Biopolymers Used as Matrices. Current Drug Delivery 2018, 15, 1360-1374, 10.2174/1567201815666180723114326.
- Rodolfo Daniel Velasco-Barraza; Ricardo Vera-Graziano; Eduardo Alberto López-Maldonado; Mercedes Teresita Oropeza-Guzmán; Syed G. Dastager; Adriana Álvarez-Andrade; Ana Leticia Iglesias; Luis Jesús Villarreal-Gómez; Study of nanofiber scaffolds of PAA, PAA/CS, and PAA/ALG for its potential use in biotechnological applications. International Journal of Polymeric Materials and Polymeric Biomaterials 2017, 67, 800-807, 10.1080/00914037.2017.1378887.
- Graciela Lizeth Pérez-González; Luis Jesús Villarreal-Gómez; Aracely Serrano-Medina; Erick José Torres-Martínez; José Manuel Cornejo-Bravo; Mucoadhesive electrospun nanofibers for drug delivery systems: applications of polymers and the parameters’ roles. International Journal of Nanomedicine 2019, ume 14, 5271-5285, 10.2147/ijn.s193328.
- Erick José Torres-Martinez; Graciela Lizeth Pérez-González; Aracely Serrano-Medina; Daniel Grande; Ricardo Vera-Graziano; Jose Cornejo-Bravo; Luis Jesús Villarreal-Gómez; Drugs Loaded into Electrospun Polymeric Nanofibers for Delivery. Journal of Pharmacy & Pharmaceutical Sciences 2019, 22, 313-331, 10.18433/jpps29674.
- Jesse Gerardo López-Covarrubias; Laura Soto-Muñoz; Ana Leticia Iglesias; Luis Jesús Villarreal-Gómez; Electrospun Nanofibers Applied to Dye Solar Sensitive Cells: A Review.. Materials 2019, 12, 3190, 10.3390/ma12193190.
- Graciela Lizeth Pérez-González; Luis Jesús Villarreal-Gómez; Amelia Olivas-Sarabia; Ricardo Valdez; José Manuel Cornejo-Bravo; Development, characterization, and in vitro assessment of multilayer mucoadhesive system containing dexamethasone sodium phosphate. International Journal of Polymeric Materials and Polymeric Biomaterials 2020, 1, 1-13, 10.1080/00914037.2020.1798433.
- Erick José Torres-Martínez; Ricardo Vera-Graziano; José Manuel Cervantes-Uc; Nina Bogdanchikova; Amelia Olivas-Sarabia; Ricardo Valdez-Castro; Aracely Serrano-Medina; Ana Leticia Iglesias; Graciela Lizeth Pérez-González; José Manuel Cornejo-Bravo; et al.Luis Jesús Villarreal-Gómez Preparation and characterization of electrospun fibrous scaffolds of either PVA or PVP for fast release of sildenafil citrate. e-Polymers 2020, 20, 746-758, 10.1515/epoly-2020-0070.
- Alan Saúl Álvarez-Suárez; Syed G. Dastager; Nina Bogdanchikova; Daniel Grande; Alexey Pestryakov; Juan Carlos García-Ramos; Graciela Lizeth Pérez-González; Karla Juárez-Moreno; Yanis Toledano-Magaña; Elena Smolentseva; et al.Juan Antonio Paz-GonzálezTatiana PopovaLyubov RachkovskayaVadim NimaevAnastasia KotlyarovaMaksim KorolevAndrey LetyaginLuis Jesús Villarreal-Gómez Electrospun Fibers and Sorbents as a Possible Basis for Effective Composite Wound Dressings. Micromachines 2020, 11, 441, 10.3390/mi11040441.
- Daniella Alejandra Pompa-Monroy; Paulina Guadalupe Figueroa-Marchant; Syed G. Dastager; Meghana Namdeo Thorat; Ana Leticia Iglesias; Valentín Miranda-Soto; Graciela Lizeth Pérez-González; Luis Jesús Villarreal-Gómez; Bacterial Biofilm Formation Using PCL/Curcumin Electrospun Fibers and Its Potential Use for Biotechnological Applications. Materials 2020, 13, 5556, 10.3390/ma13235556.
- Ahmed Barhoum; (Dr) Kaushik Pal; Hubert Rahier; Hasan Uludag; Ick Soo Kim; Mikhael Bechelany; Nanofibers as new-generation materials: From spinning and nano-spinning fabrication techniques to emerging applications. Applied Materials Today 2019, 17, 1-35, 10.1016/j.apmt.2019.06.015.
- M. R. Morad; A. Rajabi; M. Razavi; S. R. Pejman Sereshkeh; A Very Stable High Throughput Taylor Cone-jet in Electrohydrodynamics. Scientific Reports 2016, 6, 38509, 10.1038/srep38509.
- Xiangyu You; Chengcong Ye; Ping Guo; Electric field manipulation for deposition control in near-field electrospinning. Journal of Manufacturing Processes 2017, 30, 431-438, 10.1016/j.jmapro.2017.10.005.
- Masha Li; Yuansheng Zheng; Binjie Xin; Yingqi Xu; Coaxial Electrospinning: Jet Motion, Core–Shell Fiber Morphology, and Structure as a Function of Material Parameters. Industrial & Engineering Chemistry Research 2020, 59, 6301-6308, 10.1021/acs.iecr.9b05866.
- Adnan Haider; Sajjad Haider; Inn-Kyu Kang; A comprehensive review summarizing the effect of electrospinning parameters and potential applications of nanofibers in biomedical and biotechnology. Arabian Journal of Chemistry 2018, 11, 1165-1188, 10.1016/j.arabjc.2015.11.015.
- Mina Keshvardoostchokami; Sara Seidelin Majidi; Peipei Huo; Rajan Ramachandran; Menglin Chen; Bo Liu; Electrospun Nanofibers of Natural and Synthetic Polymers as Artificial Extracellular Matrix for Tissue Engineering. Nanomaterials 2020, 11, 21, 10.3390/nano11010021.
- Giuliana Gorrasi; Raffaele Longo; Gianluca Viscusi; Fabrication and Characterization of Electrospun Membranes Based on “Poly(ε-caprolactone)”, “Poly(3-hydroxybutyrate)” and Their Blend for Tunable Drug Delivery of Curcumin. Polymers 2020, 12, 2239, 10.3390/polym12102239.
- Dan-Thuy Van-Pham; Tran Thi Bich Quyen; Pham Van Toan; Chanh-Nghiem Nguyen; Ming Hua Ho; Doan Van Hong Thien; Temperature effects on electrospun chitosan nanofibers. Green Processing and Synthesis 2020, 9, 488-495, 10.1515/gps-2020-0050.