Salinity stress is one of the major threats to agricultural productivity across the globe. Research in the past three decades, therefore, has focused on analyzing the effects of salinity stress on the plants. Evidence gathered over the years supports the role of ethylene as a key regulator of salinity stress tolerance in plants. This gaseous plant hormone regulates many vital cellular processes starting from seed germination to photosynthesis for maintaining the plants’ growth and yield under salinity stress. Ethylene modulates salinity stress responses largely via maintaining the homeostasis of Na+/K+, nutrients, and reactive oxygen species (ROS) by inducing antioxidant defense in addition to elevating the assimilation of nitrates and sulfates. Moreover, a cross-talk of ethylene signaling with other phytohormones has also been observed, which collectively regulate the salinity stress responses in plants.
- ROS
- ethylene
- antioxidants
- salinity stress
- photosynthesis
- programmed cell death
- seed germination
- hormone cross-talk
1. Introduction
2. Ethylene is a Key Modulator of Salinity Stress Responses in Plants
Ethylene biosynthesis and signaling are implicated in salinity stress tolerance in plants (Table S1). Overproduction of endogenous ethylene or exogenous treatment of ethylene-releasing compounds such as ethephon or ethylene precursors such as 1-aminocyclopropane-1-carboxylic acid (ACC) increase salinity stress tolerance in various plants including Arabidopsis [37][47] and maize [12]. Moreover, ethylene has also been found as an essential positive mediator of salinity stress tolerance in grapevine [13], maize [12], and tomato [38][48]. Promising evidence of the involvement of melatonin in enhancing salinity stress tolerance by promoting MYB108A-mediated ethylene biosynthesis was also reported in grapevines [13]. It has been reported that inhibition of ethylene biosynthesis and/or signaling leads to increased sensitivity of plants to salinity stress. Gharbi et al. [38][48] hypothesize that ethylene exhibits a positive effect in adaptation to salinity stress probably by maintaining stomatal conductance, water use efficiency, and osmotic adjustment in Solanum chilense [38][48]. Calcium carbide (CaC2), a precursor of acetylene exhibiting similar effects to ethylene, is commonly used to improve seed germination rates and ethylene concentration in germinating seeds under salinity stress. It is proposed that CaC2 is involved in osmotic adjustments and management of oxidative stress by increasing solute concentrations and activities of antioxidant enzymes in germinating seeds. CaC2 considerably enhances the activity of SOD and CAT besides reducing the H2O2 and malondialdehyde (MDA) concentrations for improving seed germination in Cucumis sativus under salinity stress conditions [39][49]. These reports collectively suggest a positive regulatory role of ethylene in salt stress tolerance in plants; however, negative regulation of ethylene in salinity stress response has also been reported in many plants, including rice. Transgenic plants with reduced ethylene biosynthesis showed elevated salinity tolerance in tobacco [40][50]. Similarly, exogenous treatment with ethylene in rice resulted in salinity hypersensitivity [41][42][51,52]. Elevation of ethylene production under salinity stress significantly reduced growth, grain filling, and development of spikelets in rice [43][53]. Exogenous application of 1-MCP, an ethylene action inhibitor, to the rice spikelets resulted in improved physiological, agronomical, and biochemical characteristics under salinity stress, further suggesting a negative role of ethylene in salinity stress tolerance in rice [44][54]. Gene mutation and transgenic analyses have shown that almost all the components of ethylene biosynthesis and signal transduction pathway respond to salinity stress either positively or negatively. In cotton, short- and long-term salinity stress resulted in upregulation of different sets of genes of ethylene signaling involved in the regulation of salinity stress [45][55]. These genes include (a) ethylene biosynthesis genes (homologs of ACS1, ACS12, ACO1, and ACO3), (b) ethylene receptor genes (ETR1, ETR2, and EIN4), (c) ethylene signaling pathway (ERF1, ERF2, EIN3, and MEKK1-MKK2-MPK4/6 kinases), and (d) feedback mechanism gene (CTR1) [45][55].2.1. Salinity Stress and Ethylene Receptors
2.2. Salinity Stress and EIN Proteins
2.3. Effects of Salinity Stress on ERFs and other Ethylene-Responsive Transcription Factors
3. Seed Germination Regulated by Ethylene under Salinity Stress
Successful seed germination is the most crucial phase in the initiation of the life cycle of plants and is regulated by many external and internal factors including phytohormones, light, temperature, drought, and salinity [79][80][81][82][88,89,90,91]. Seed germination is severely affected in saline soil, which negatively influences plant growth and crop yield [10] (Figure 1). Different components of ethylene signaling participate either positively or negatively during seed germination and seedling growth under salinity stress [83][92]. For example, seed germination in Arabidopsis was inhibited by ETR1 and EIN4, whereas ETR2 was found to be a positive regulator involved in stimulating seed germination during salinity stress conditions [49][59].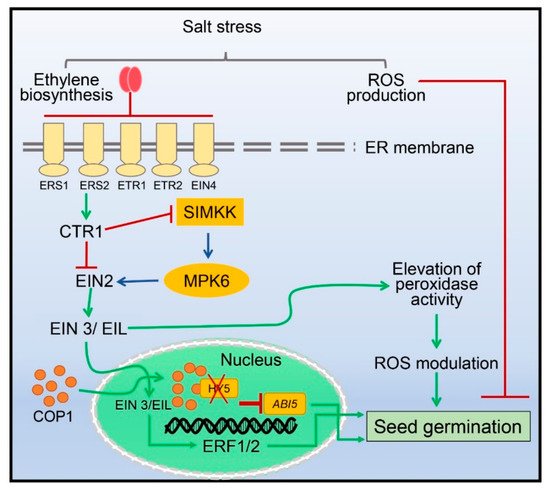
4. Fine-Tuning of Photosynthetic Machinery by Ethylene during Salinity Stress
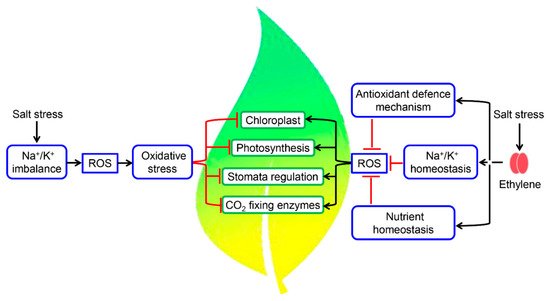
Homeostasis of essential elements like N, P, K, S, and Ca is altered during salinity stress, which in turn affects the photosynthetic efficiency of plants [99][100][101][108,109,110]. Salinity stress induces oxidative stress through increased production of ROS, which can disrupt chloroplast functions (Figure 2). Salts at higher concentrations induce both osmotic and ionic stresses, which affect photosynthetic activity either by closing the stomata or by reducing the activity of CO2-fixing enzymes and availability of water in the plant cells [102][111] (Figure 2). Activities of CO2-fixing enzymes are reduced at higher concentrations of Na+, and tolerance of these enzymes to Na+ concentrations varies from species to species [103][112]. Na+ ions imbalance the proton motive force and thus influence photosynthetic machinery and chloroplastic functions [104][113]. Salinity stress influences the photosynthetic parameters including chlorophyll, photosystems, net photosynthesis rate (Pn), chlorophyll fluorescence parameters, soluble sugar contents, and ribulose bisphosphate carboxylase/oxygenase (RuBisCO) activity [105][114]. Recently, it was reported that tomato plants showed improved photosynthesis, metabolic homeostasis, and growth rate as a result of elevated CO2 under salinity stress by decreasing the amount of ABA hormone and ACC [106][115]. Among all the photosynthetic parameters, photosystem II (PSII) is the most susceptible to various abiotic stresses including salinity [107][108][116,117]. Homeostasis of Na+ ions maintain membrane integrity, relative water content, net photosynthesis, and yield. Ethylene has been shown to promote the homeostasis of Na+/K+, nutrients, and ROS to enhance plant tolerance to salinity [57][40]. The ctr1-1 mutants maintain relatively higher concentrations of K+ and lower concentrations of Na+ in contrast to ein2-5 or ein3 plants, where an opposite trend of K+ and Na+ concentration was observed compared with the WT undertreated and optimum conditions [53][63]. Because of this altered K+ and Na+ homeostasis, ctr1-1 plants displayed a slight reduction in leaf area and root elongation, while ein2-5 or ein3-1 mutants showed magnified retardation in plant growth compared to the WT under salinity stress [53][63]. In pomegranate, salinity decreased the net photosynthetic rate, chlorophyll content, stomatal conductance, relative water content, and electrical conductivity [105][109][110][114,118,119]. Further heat map analysis showed that antiapoptotic genes BAG6 and BAG7 were clustered together with ERS2, EIN3, and ACS and that the transcripts levels of BAG6, BAG7, ERS2, and ACS2 were significantly suppressed in the response to salinity. The inclusion of ACC or ethylene source in the saline solution restored the expression levels of BAG6 and BAG7, suggesting the involvement of ethylene in the regulation of these antiapoptotic genes under salinity stress [111][120].