Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Vivi Li and Version 4 by Vivi Li.
Melleins are 3,4-dihydroisocoumarins mainly produced by fungi, but also by plants, insects and bacteria. These specialized metabolites play important roles in the life cycles of the producers and they are involved in many biochemical and ecological processes.
- melleins
- chemical characterization
- biological activities
- biosynthesis
- 3
- 4-dihydroisocoumarins
1. Introduction
Melleins are a subgroup of 3,4-dihydroisocoumarins. In general, these secondary metabolites belong to the class of polyketides. The 3,4-dihydroisocoumarins are also a subgroup of the isocoumarins, a well-known polyketides family that is the structural isomer of coumarin. The general moieties of these four groups of natural occurring compounds are reported in Figure 1, and their IUPAC names are chromen-2-one, 1H-isochromen-1-one, isochroman-1-one and 8-hydroxy-3-methylisochroman-1-one, respectively.
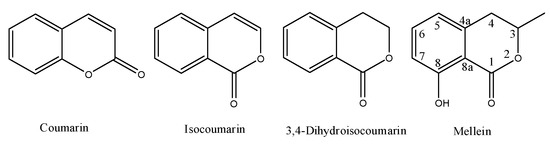
Figure 1. Structure of coumarin, isocoumarin, 3,4-dihydroisocoumarin and mellein.
The first coumarin was obtained as natural compound from Coumarouna odorata (tonka tree) [1], which is a species of flowering tree in the pea family (Fabaceae). The first report on 3,4-dihydroisocoumarin was in 1916 when the hydrangenol was isolated from Hydrangea hortensia, a species of flowering plants native to Asia and the Americas [2]. However, mellein is the best known in this subgroup, although previously it was named ocracin when isolated from the fungus Aspergillus melleus on 1933 [3]. Coumarin and isocoumarin derivatives are produced by bacteria, fungi, higher plants, insects, lichens, liverworts, and marine sponges. They showed different biological activities, such as antimicrobial, antitumor, antileukemic, and antiviral ones. Furthermore, they also exhibited toxicity as ochratoxin A, which is a mycotoxin, biosynthesized by Aspergillus and Penicillium species, which usually contaminates a variety of food imparting heavy toxicity against animals and humans [4]. The isolation of several coumarins, isocoumarins and 3,4-dihydroisocoumarins from different natural sources, and their important biological activities were covered by some previous reviews [5][6][7]. Some other reviews extensively describe the reaction sequences applied over the year for their synthesis [8][9][10].
2. Natural Sources, Isolation, Chemical Characterization and Biological Activities
2.1. Melleins from Fungi
Fungi are the most important source of melleins, and (R)-(-)-mellein (1, Figure 2) is the most common among this group.
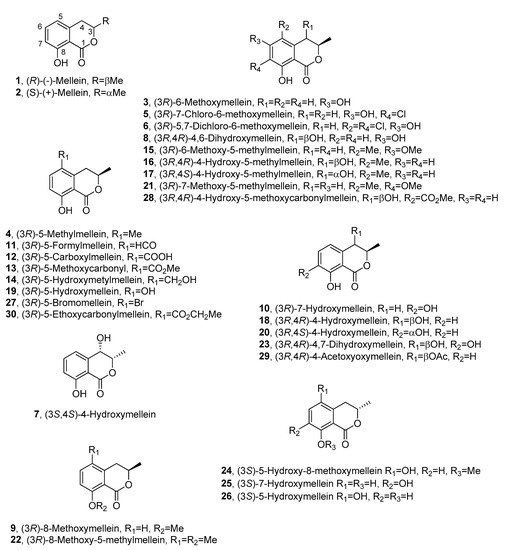

Figure 2. The structures of 1–30.
Compound 1 was isolated for the first time in 1933 from the fungus Aspergillus melleus [3] and named ocracin, as cited above. However, its structure was determined only in 1955 [11] and the R absolute configuration (AC) at C-3 was successively assigned [12][13]. Its enantiomer, the (S)-(+)-mellein (2, Figure 2), is also known as a natural product, but it is produced by few species of fungi compared with 1. In particular, compound 2 was firstly isolated from an unidentified fungus [14] and then from the cultures of the insect pathogen Fusarium larvarum, together with five other secondary metabolites [15].
A structural analogue of 1 and 2 was isolated from Sporormia bipartis and characterized as 6-methoxymellein (3, Figure 2) [16]; another analogue was isolated from the plant pathogen Fusicoccum amygdali, together with the main phytotoxic metabolite fusicoccin A. The compound, whose structure was elucidated by NMR, was found to be 5-methylmellein (4, Figure 2). In this study, 4 inhibited conidia germination in some fungi, but it had no detectable phytotoxicity in vitro [17]. Compound 3 was obtained as the main metabolite produced by the fungus Sporormia affinis together with two chlorinated analogues, which were characterized as 7-chloro-6-methoxymellein (5, Figure 2) and 5,7-dichloro-6-methoxymellein (6, Figure 2) by NMR and mass spectra [18].
Studies carried out to evaluate the production of mycotoxins by Aspergillus oniki 1784, allowed to isolate 1 together with two related compounds, which were characterized as 3-methyl-4,8-dihydroxy-3,4-dihydroisocoumarin and 3-methyl-3,8-dihydroxy-3,4-dihydroisocoumarin. Their LD50 values for mice were measured by intraperitoneal injection, but their stereochemistry was not determined [19]. Successively, compound 1 together with cis-4-hydroxymellein and other five metabolites were isolated from Lasidiplodia theobromae culture filtrates, which inhibited the green plant growth. However, for 1 and its analogues, no biological activity was reported [20]. One of the stereoisomers of 4-hydroxymellein was also isolated together with 1 from Aspergillus ochraceus [21][22]. The AC of one of the two enantiomers of cis-4-hydroxymellein was determined when it was purified from the mycelium of Cercospora taiwanensis, together with 2, and characterized as cis-(3S,4S)-4-hydroxymellein (7, Figure 2) [23]. Furthermore, a 4,6-dihydroxymellein (8, Figure 2) was isolated in a screening of 61 other species of Cercospora [24].
(R)-(-)-mellein (1), (R)-8-methoxymellein (9, Figure 2) and (3R)-7-hydroxymellein (10, Figure 2) were isolated from Septoria nodorum by Devys and co-authors and characterized by NMR spectroscopy [25].
A series of dihydroisocoumarin derivatives were isolated in a systematic study on metabolites of the Xylariaceous fungi belonging to Hypoxylon and Numularia species. 5-methylmellein (4) was produced by almost all species studied, whereas 1 was produced by Hypoxylon fragiforme, Hypoxylon howeianum, Hypoxylon haematostroma, Hypoxylon venusfuissimum and Hypoxylon deustum. In the same study, five other analogues of mellein were isolated and identified as 5-formyl- (11, Figure 2), 5-carboxy- (12, Figure 2), 5-methoxycarbonyl (13, Figure 2), 5-hydroxymethyl (14, Figure 2), and 6-methoxy-5-methyl- (15, Figure 2) mellein. 11 was produced by Numularia discreta and Numularia broomiana, 12 by Hypoxylon mammatum, Hypoxylon illitum and N. discreta, 13 by H. mammatum, 14 by H. illitum and 15 by Hypoxylon atropunctatum [26].
Mellein 5-carboxylic acid (12), together with 5-methyl mellein (4), (S)-(+)-mellein (2) and other compounds, was isolated from Phomopsis oblonga in a study for the production of elm bark beetle boring and feeding deterrents. Compound 2 was obtained as minor metabolite, while 4 and 12 were active against adult females of Scolytus sp. beetles [27]. The AC at C-3 of (-)-5-carboxylmellein (12) and (-)-5-hydroxylmethylmellein (15) was assigned as R by chemical correlations when they were isolated from Valsa ceratosperma, a fungus inducing apple canker. The same fungus also synthesizes (-)-5-methylmellein (4), and two new compounds which were characterized as cis-(3R,4R)-(-)-4-hydroxy-5-methylmellein (16, Figure 2) and trans-(3R,4S)-(+)-4-hydroxy-5-methylmellein (17, Figure 2). All the five compounds were phytotoxic to apple shoots and lettuce seedlings [28].
The first report of 1 in the genus Nectria occurred in 1986 when it was isolated from Nectria fuckeliana by Ayer and Shewchuk [29].
Five phytotoxic metabolites were purified from the culture filtrates of Botryosphearia obtusa (Schw.) Shoemaker, the causal agent of frogeye leaf spot and fruit black rot of apple. Among them, (R)-(-)-mellein (1), cis-(3R,4R)-(-)-4-hydroxymellein (18, Figure 2) and (3R)-5-hydroxymellein (19, Figure 2) were identifiedusing spectroscopic and optical methods [30]. 19 was isolated for the first time as a fungal metabolite and the phytotoxicity of all metabolites was tested by leaf puncture assay on seventeen apple cultivars and eight weed species. The apple cultivars Supergold and Silverspure and the weeds species prickely sida and morning glory were the most sensitive species to all the phytotoxins [31]. Successively, (R)-(-)-mellein (1) was isolated as a phytotoxic metabolite, together with citrinin, tyrosol and α–acetylorcinol, from Stagonospora apocynin, which caused leaf spot disease on hemp dogbane (Apocynum cannabinum L.). All the compounds were non–specific toxins causing necrosis when assayed on hemp dogbane leaves, and those of eight other weed species [32].
In a study carried out to select in vitro wheat embryos with a high level of resistance, 1 was identified in the fungal organic extract of Septoria nodorum (Berk.) [33]. (3R,4S)-(-)-4-hydroxymellein (20, Figure 2), together with (3R,4R)-(-)-4-hydroxymellein (18), was isolated for the first time from the same organic extract [34]. Phoma tracheiphila, the fungus responsible for “mal secco” citrus disease, also produced (R)-(-)-mellein (1). Compound 1 was phytotoxic when tested at a concentration of 100 mg/mL in tomato cuttings and caused 100% mortality of Artemia salina larvae at 200 mg/mL [35].
Compound 1 was isolated together with other four compounds in a study conducted on 85 Pezicula strains isolated as endophytes from living branches of ten deciduous and coniferous trees. All the compounds demonstrated strong fungicidal and herbicidal activity, and to a lesser extent, algicidal and antibacterial activity. Their production was taxonomically significant [36].
(R)-(-)-mellein (1), (3R,4R)-hydroxymellein (18) and (3R,4S)-hydroxymellein were also isolated from Microsphaeropsis sp. in a study conducted on some fungi obtained from marine sponges. In the same study (3R)-6-methoxymellein (3) and (3R)-6-methoxy-7-chloromellein (5) were also isolated from the culture extracts of the Coniothyrium sp. All these mellein analogues showed antifungal activity against Eurotium repens and Ustilago violacea [37].
A crude extract of Aspergillus ochraceus inhibited the final stage of hepatitis C virus (HCV) replication. A bio-guided purification of the extract afforded the known (R)-(-)-mellein (1), together with circumdatins G and F, which were identified by NMR spectroscopy. Compound 1 inhibited HCV protease with an IC50 value of 35 μM [38].
Two novel melleins, namely, 7-methoxy-5-methylmellein (21, Figure 2) and 8-methoxy-5-methylmellein (22, Figure 2), together with three known dihydroisocoumarins and a tetralone derivative, were isolated from a culture filtrate of Cytospora eucalypticola. Their structures were elucidated by spectroscopic methods. Compounds 21 and 22 showed moderate antifungal and antibacterial activity against Gram-positive bacteria [39].
Apiospora montagnei, a marine fungus, isolated from the North Sea alga Polysiphonia violacea, produced a plethora of secondary metabolites comprising (R)-(-)-mellein (1) and (R)-8-methoxymellein (9) [40]. Two novel compounds, namely, the monomethyl ester of 9-hydroxyhexylitaconic acid and the (-)-enantiomer of the known (+)-hexylitaconic acid, were also isolated and characterized by spectroscopic methods. The crude fungal extract and all the metabolites did not show antibacterial, antifungal and antialgal activity [40].
Sphaeropsis sapinea was isolated from declining pine (Pinus radiata) plants in Sardinia and studied for its ability to produce phytotoxic metabolites [41]. S. sapinea was grown in liquid culture and the purification of the corresponding organic extract afforded the three already known (R)-(-)-mellein (1), (3R,4R)-4-hydroxymellein (18) and (3R,4S)-4-hydroxymellein (20) isolated for the first time from this fungus. When assayed for phytotoxic and antifungal activities on host and non-host plants and on some phytopathogenic fungi, 1 was the most active compound, while 18 and 20 showed only a synergic effect in both tests [41]. The same melleins were isolated from Botryosphaeria mamane PSU-M76, along with other three known secondary metabolites and a dihydrobenzofuran derivative named botryomaman [42]. Their antibacterial activity against Staphylococcus aureus ATCC 25923, S. aureus SK1 and compounds 1, 18 and 20 was tested; they were inactive with equal MIC values of > 128 μg/mL [42].
Among a collection of 250 foliar endophytes of Picea glauca, several isolates produced toxic metabolites against Choristoneura fumiferana (spruce budworm) [43]. Three of them (strains CBS 120381, CBS 120379 and CBS 120380) were selected for isolation and characterization of phytotoxic metabolites by LC-MS and LC-NMR. The DNA sequencing revealed that CBS 120381 belongs to the Xylariaceae and it was near but not identical to Nemania serpens. The sequencing data indicated that CBS 120379 and CBS 120380 were both species of Lophodermium, with CBS 120379 most like fungi in the Rhytistimataceae. Two of them produced at least one mellein. CBS 120379 synthesized (R)-(-)-mellein (1), while CBS 120381 produced three known melleins: 5-methyl- (4), 5-formyl- (11) and 5-methoxycarbonyl- (13) mellein [43].
Botryosphaeria obtusa, the teleomorph of Diplodia seriata, is a pathogen associated with grapevine Botryosphaeria dieback. The fungus was studied for its ability to synthesize phytotoxic metabolites, which were purified and characterized by spectroscopic data [44]. The three known melleins, (R)-(-)-mellein (1), (3R)-7-hydroxymellein (10) and (3R,4R)-4-hydroxymellein (18), and a novel mellein characterized as 4,7-dihydroxymellein (23, Figure 2) were isolated from the liquid culture of this fungus [44]. When assayed on grapevine leaves, 23 was the most active compound, inducing full leaf necrosis with minimum inhibitory concentrations (MIC) of 2 μg/mL [44]. Other Botryosphaeriaceae, such as Bothryosphaeria rhodina PSU-M35 and PSU-M114, were studied for secondary metabolite production because they showed antibacterial activity against S. aureus, both standard ATCC 25922 (SA) and methicillin-resistant (MRSA) strains. (R)-(-)-mellein (1), (3R,4R)-4-hydroxymellein (18), (3R,4S)-4-hydroxymellein (20) and (3R)-5-hydroxymellein (19) were recognized by spectroscopic and physic properties. Compounds 1, 18, 19 and 20 showed good activity against both strains; however, the most active compound isolated was (3S)-lasiodiplodin [45].
Tubercularia sp. TF5 was isolated from the bark of Taxus mairei collected in Fujian Province, southeast China. Taxol, the well-known anticancer compound, was produced by this strain. Thus, it was studied for the production of other bioactive metabolites. The chromatographic purification of the culture filtrate extract yielded 5-carboxymellein (12) which was identified by spectroscopic data [46].
Neofusicoccum parvum is the one of the most virulent Botryosphaeriaceae species causing heavy grapevine trunk diseases. (3R,4R)-4-hydroxymellein (18), (3R,4S)-4-hydroxymellein (20), isosclerone and tyrosol were produced by this fungus and identified by spectroscopic methods. All the compounds were tested to evaluate their phytotoxic activities on tomato cuttings at different concentrations, and they showed toxicity ranging from slight to drastic leaf wilting, and compound 18 showed the highest phytotoxicity [47]. Diplodia africana, the causal agent of branch dieback on Juniperus phoenicea, belonging to the same fungal family of the Botryosphaeriaceae, produced phototoxic metabolites [48]. Two novel phytotoxic dihydrofuropyran-2-ones, named afritoxinones A and B, were isolated, together with known compounds, from the culture filtrates of D. africana. Besides afritoxinones A and B, the three well-known melleins (R)-(-)-mellein (1), (3R,4R)-4-hydroxymellein (18) and (3R,4S)-4-hydroxymellein (20) were isolated [48].
Endophytic fungi living in the intracellular spaces of plants are able to synthesize novel bioactive secondary metabolites. Indeed, the endophytic fungal strain BCRC 33717, obtained from the bark of the medicinal plant Cinnamomum sp., produced a plethora of secondary metabolites, including four melleins, which were recognized as the 5-formyl- (11), 5-carboxy- (12) and 5-hydroxy- (19) mellein, and the novel (3S)-5-hydroxy-8-O-methylmellein (24, Figure 2) [49]. Their structures were determined by NMR and MS methods [49]. Annulohypoxylon squamulosum BCRC 34022, obtained from the same medicinal plants, when grown on a long-grain rice fermented culture, synthesized eleven different metabolites, which were extracted with n-BuOH-soluble 95% EtOH. Among the secondary bioactive metabolites purified from these extracts, three melleins were characterized by spectroscopic methods as(+)-(S)-mellein (2), (S)-7-hydroxymellein (25, Figure 2), and (S)-5-hydroxymellein (26, Figure 2). [50]. Compounds 25 and 26 were isolated for the first time and are the enantiomers of the previously reported compounds 10 and 19. When assayed on MCF-7 (human breast adenocarcinoma), NCIH460 (non-small-cell lung cancer) and SF-268 (glioblastoma cells), compound 2 possessed moderate toxicity against MCF-7, NCI-H460 and SF-268 cancer cell lines, while 25 and 26 showed weak to negligible activities against all the three cell lines [50].
To keep talking about endophytic fungi, Epicoccum nigrum, an ascomycete fungus distributed worldwide, colonizes different types of soils and host plants, and was used as a biocontrol agent for plant pathogens. E. nigrum wild type P16 produced secondary metabolites, including (R)-(-)-mellein (1) [51]. Three E. nigrum agro-transformants, namely, P16–17, P16–47set and P16–91, were studied in order identify the genes related to the synthesis of a new natural compound produced by E. nigrum P16. The comparison of the extracts of the wild type and the transformants by GC-MS, revealed that the mutants were capable of producing (R)-5-hydroxymellein (19) as well [51].
Biscogniauxia nummularia (Bull.), a seemingly endophytic fungus isolated from the plum yew Cephalotaxus harringtonia, produced a new guaiane sesquiterpene together with a previously known terpenoid, xylaranol B, and two mellein derivatives identified as 5-methylmellein (4) and 8-methoxy-5-methylmellein (22) [52].
Seimatosporium sp. was studied for its ability to synthesize biologically active compounds in a program planned to investigate endophytic fungi for new products for pharmacy and plant protection [53]. (R)-(-)-Mellein (1), (3R,4R)-4-hydroxymellein (18), (3R,4S)-4-hydroxymellein (20), (3R)-5-hydroxymethylmellein (14) and (3R,4S)-4-hydroxy-5-methylmellein (17) were isolated from fermentation extracts of Seimatosporium sp. and identified by spectroscopic methods [53].
Two novel succinic acid derivatives, xylacinic acids A and B, were isolated, along with (R)-8-methoxymellein (9), (3R)-5-methoxycarbonylmellein (13) and (3R)-5-hydroxymellein (19), from the mangrove-derived fungus Xylaria cubensis PSU-MA34 [54]. Al the metabolites isolated were tested for antibacterial activity against S. aureus ATCC 25923 and MRSA and for cytotoxicity against KB cells; however, the melleins were inactive [54].
The extracts from eleven fungal strains associated with Eudistoma vannamei were assayed against two cell lines asthe colon and melanoma cancer cell lines [55]. Aspergillus sp. yielded the most promising extract. Its potato dextrose broth extract was purified by bio-guided chromatographic method, and (R)-(-)-mellein (1), (3R,4R)-4-hydroxymellein (18) and (3R,4S)-4-hydroxymellein (20) were isolated, along with penicillic acid. All isolated compounds were tested for their cytotoxicity; however, only penicillic acid showed cytotoxic activity (cell growth inhibitions > 95%) [55].
The bioactive metabolites were produced by an endophytic fungus, identified as Nigrospora sp. by rDNA sequence analysis, and four of them were identified by comparison of the 1H-NMR and 13C-NMR spectroscopic data with those previously reported. Among them, (R)-(-)-mellein (1) was identified and showed only weak antifungal activity [56]. In another investigation the crude extracts from cultures of Pezicula livida were studied for larvicidal activity. The bio-guided chromatographic purification of the extract also afforded (R)-(-)-mellein (1), and its larvicidal activity was reported the first time with an LC50 value of 1.4 ppm against Aedes aegypti [57].
An interesting study was conducted by Yun and co-authors (2013), who added NaBr to the fermentation of the fungus Aspergillus ochraceus [58]. This resulted in the production of a new brominated mellein analogue; namely, (3R)-5-bromomellein (27, Figure 2). Furthermore, three known compounds, (R)-(-)-mellein (1), clavatol and circumdatin A, were also recognized in the same culture. The structure of 27 was assigned through spectroscopic data analyses. All the isolated compounds showed mild radical scavenging activity against 1,1-diphenyl-2-picrylhydrazyl (DPPH) [58].
The organic extract from culture broths of Arthrinium state of Apiospora montagnei afforded the main metabolites, which were identified as (R)-(-)-mellein (1) and (3R,4R)-4-hydroxymellein (18) according to their spectroscopic and physical data. In addition, their activity against Schistosoma mansoni (adult worms) was tested. Despite the structural similarity, 1 caused the death of 100% of parasites (both male and female) at 200 μg/mL, whereas compound 18 caused the death of 50% of adult worms at 12.5 μg/mL and 100% at 50 μg/mL [59].
Xylaria sp. PSU-G12, isolated from Garcinia hombroniana, when grown in liquid culture, yielded a corresponding organic extract displaying antioxidant activity [60]. A plethora of secondary metabolites were isolated and characterized by spectroscopic methods, and among them a novel mellein analogue was isolated and characterized as (3R,4R)-5-carbomethoxy-4-hydroxymellein, and then named xylarellein (28, Figure 2). Three known melleins were also identified as (3R)-5-methylmellein (4), (3R)-5-carboxylmellein (12) and (3R)-5-methoxycarbonilmellein (13). All the isolated compounds were evaluated for antioxidant activity in DPPH assays, but none of them exhibited antioxidant activity, highlighting the possibility that the antioxidant activity of the crude extract may involve a synergistic effect [60].
Two formerly undescribed polyketide metabolites were obtained from the cultures of an endophytic fungus isolated from Meliotus dentatus. The two compounds appeared to be, based on spectroscopic data, a new mellein named cis-4-acetoxyoxymellein (29, Figure 2) and one of its derivatives [61]. The two compounds were tested in an agar diffusion assay for their antifungal, antibacterial and algicidal activities against Botrytis cinerea, Septoria tritici, Phytophthora infestans, Microbotryum violaceum, Escherichia coli, Bacillus megaterium and Chlorella fusca. Both metabolites displayed strong antibacterial activity, especially towards Escherichia coli and Bacillus megaterium. In addition, both displayed algicidal activity against C. fusca and good antifungal activity against M. violaceum, B. cinerea and S. tritici [61].
The endophyte Xylaria sp., isolated from a surface-sterilized Concord grape leaf (Vitis labrusca), grown in liquid culture, produced seven compounds, among which two mellein derivatives were isolated and identified by spectroscopic data as (3R)-5-methoxycarbonylmellein (13) and (3R)-5-carboxylmellein (12). However, no biological activities were tested for these two metabolites [62].
(R)-(-)-mellein (1) was also produced by Lasiodiplodia sp. ME4-2, an endophytic fungus obtained from the floral sections of Viscum coloratum [63], and from Pezicula sp., an endophyte obtained from twigs of Forsythia viridissima, Zhejiang Province, Southeast China [64]. In this latter study, (R)-(-)-mellein (1) showed growth inhibition against nine plant pathogenic fungi, especially Botrytis cinerea and Fulvia fulva with EC50 values below 50 μg/mL [64].
From the solid culture of the endophytic fungus Xylaria sp. SNB-GTC2501, which was obtained from the leaves of Bisboecklera microcephala, the two already known mellein derivatives 4 and 12 were obtained, whose antimicrobial potential was assessed against human pathogens (i.e., Staphylococcus aureus, Trichophyton rubrum and Candida albicans). However, their minimal inhibitory concentrations were more than 128 μg/mL. In addition, 4 and 12 were not cytotoxic towards MRC5 cells (IC50 >100 μM) [65].
(3R)-5-methylmellein (4), (3R)-5-formylmellein (11) and (3R)-5-carboxymellein (12), were also synthesized, together with two undescribed dihydronaphthalenones and the known 2,6-dihydroxy-4-methylacetophenone, by the endophytic fungus Nodulisporium sp., isolated from Antidesma ghaesembilla. No biological activities were reported for the mellein derivatives 4, 11 and 12 [66].
The endophytic fungus Botryosphaeria sp. KcF6 derived from the mangrove plant Kandelia candel produced (3R)-8-methoxymellein (9) and (3R,4S)-4-hydroxylmellein (20), which were identified by spectroscopic data [67]. The isolated compounds were evaluated for their cytotoxic and anti-inflammatory (COX-2) activities, but 9 and 20 were inactive [67].
The previously undescribed (3R)-5-ethoxycarbonylmellein (30, Figure 2) was isolated from the fungus Marasmiellus ramealis isolated in China together with the known 13 and other nine compounds. The structure of the new compound was elucidated by spectroscopic methods, but no activity was reported [68].
The ability of N. parvum to produce phytotoxins was further investigated by Abou-Mansour et al. [69]. (R)-(-)-mellein (1), (3R,4R)-4-hydroxymellein (18) and (3R,4S)-4-hydroxymellein (20), already reported as phytotoxic metabolites produced by another strain of N. parvum [47], were isolated together with 3-hydroxymellein and other compounds belong to different chemical families. 3-Hydroxymellein (31, Figure 3) was previously isolated from Aspergillus oniki 1784 [19], but this study provided its first 1H and 13C NMR data. Its 3R AC was assigned by comparing its CD spectrum with that of 1.
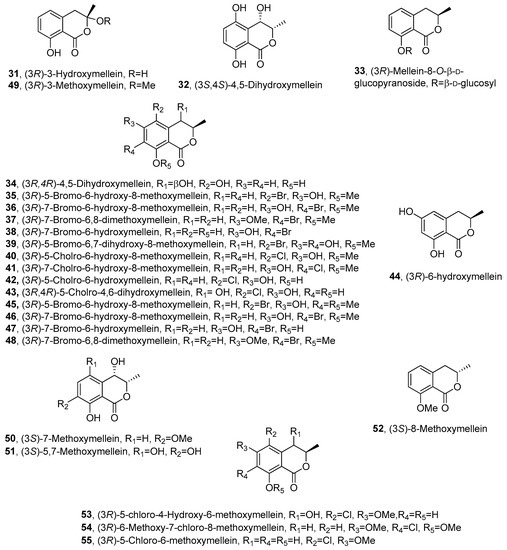

Figure 3. The structures of compounds 31–55.
The phytotoxicity of the main isolated compounds were assessed 48 h after-inoculation at concentrations of 100 and 200 μg/mL, on leaf discs of Vitis vinifera cv. Chardonnay. All the compounds tested induced necrosis on host plant leaves, and among the four melleins, (3R)-3-hydroxymellein (31) was the most active at the lower concentration [69].
Six species of Lasiodiplodia isolated in Brazil and causing Botryosphaeria dieback of grapevines were studied to evaluate their production of phytotoxic secondary metabolites. Some mellein derivatives were produced, as ascertained by LC/MS. In particular, Lasiodiplodia brasiliense synthesized (3R,4S)-4-hydroxymellein (20), and Lasiodiplodia euphorbicola produced (R)-(-)-mellein (1), (3R,4R)- and (3R,4S)- 4-hydroxymellein (18 and 20) [70].
The production of 1 by the endophyte Lasiodiplodia theobromae was confirmed in a study on the metabolomics-guided isolation of anti-trypanosomal metabolites. However, 1 was inactive when tested against Trypanosoma brucei brucei [71]. Compound 1 was also isolated together tyrosol and a new isochromanone, named fraxitoxin, from liquid cultures of Diplodia fraxini, a pathogen involved in the etiology of canker and dieback disease of Fraxinus spp. in Europe [72].
Paraconiothyrium sporulosum YK-03, a marine-derived fungus, also produced 1 together with seven mellein derivatives, including the previously undescribed (3S,4S)-4,5-dihydroxymellein (32, Figure 3) and (R)-(-)-mellein-8-O-β-D-glucopyranoside (33, Figure 3). Their structures and ACs were determined by comprehensive spectroscopic and computational electronic circular dichroism (ECD). Furthermore, their monosaccharide composition was determined by HPLC coupled with optical rotation detector. The known compounds were identified by spectroscopic methods as (3R)-7-hydroxymellein (10), (3R)-5-hydroxymellein (19), (3R,4R)-4-hydroxymellein (18), (3R,4S)-4-hydroxymellein (20) and (3R,4S)-4,5-dihydroxymellein (34, Figure 3) [73]. Compound 34 was previously isolated from the marine fungus Phomopsis sp. (number ZH-111) [73]. All the compounds were assessed for their cytotoxic activities against the human cancer cell lines A549 and MCF-7. However, none of them showed meaningful cytotoxicity against these two cell lines [74].
The endophytic fungus Lachnum palmae, obtained from Przewalskia tangutica, was exposed to a histone deacetylase inhibitor SAHA and produced seven previously undescribed halogenated dihydroisocoumarins, named palmaerones A-G (35-41, Figure 3), together with eleven known dihydroisocoumarins. They were identified by spectroscopic and optical methods as 1, 3, 18, 19, 20, (3R)-5-bromo-6-hydroxy-8-methoxymellein (35), (3R)-7-bromo-6-hydroxy-8-methoxymellein (36), (3R)-7-bromo-6,8-dimethoxymellein (37), (3R)-7-bromo-6-hydroxy-mellein (38), (3R)-5-bromo-6,7-dihydroxy-8-methoxy-mellein (39), (3R)-5-chloro-6-hydroxy-8-methoxy-mellein (40), (3R)-7-chloro-6-hydroxy-8-methoxy-mellein (41), (3R)-5-cholro-6-hydroxymellein (42, Figure 3), (3R,4R)-5-cholro-4,6-dihydroxymellein (43, Figure 3), (3R)-6-hydroxymellein (44, Figure 3), (3R)-5-bromo-6-hydroxy-8-methoxy-mellein, named palmaerone A (45, Figure 3), (3R)-7-bromo-6-hydroxy-8-methoxy-mellein, named palmaerone B (46, Figure 3) and (3R)-7-bromo-6-hydroxy-mellein, named palmaerone D (47, Figure 3) [75]. The three melleins 45-47 were previously isolated from the same fungus together with the other mellein derivative, namely, (3R)-7-bromo-6,8-dimethoxy-mellein, which was named palmerin C (48, Figure 3) [76]. Compounds 35-41 showed antimicrobial activities against the strains of Candida neoformans, Penicillium sp., Candida albicans, Bacilus subtilis and S. aureus, and compound 39 showed potential antimicrobial activity against all the test strains with the MIC value in the range of 10–55 μg/mL. Metabolites 35 and 39 exhibited mild inhibitory effects on nitric oxide (NO) production in lipopolysaccharide (LPS)-induced RAW 264.7 cells, with the IC50 values of 26.3 and 38.7 μM, respectively and no evident toxicities were observed at 50 μM. Compound 39 showed weak cytotoxicity against HepG2 with the IC50 value of 42.8 mM [76].
An interesting study was carried out on 579 fungal culture extracts to identify the bioactive compounds capable of inhibiting histone deacetylase activity of Sirtuin A (SirA), produced by the fungus Aspergillus nidulans [77]. Sirtuin is a family of histones that are implicated in fungal growth and secondary metabolite production. Eight fungal strains, belonging to different families, showed to produced SirA inhibitors, and among them, Didymobotryum rigidum JCM 8837 showed to produce 5-methylmellein (4). This mellein derivative was found to inhibit SirA activity with IC50 of 120 μM. In addition, in another experiment compound 4 was added to A. nidulans cultures. This resulted in an increased secondary metabolite production. In conclusion, the results highlighted that 5-methylmellein (4) can modulate fungal secondary metabolism and is a potential tool for screening novel compounds derived from fungi [77].
Compound 1 was isolated from the culture filtrates of Diplodia mutila FF18 Diplodia seriata H141a, Neofusicoccum australe VP13 and Neofusicoccum luteum, as resulted from a recent study on phytotoxic metabolites produced by nine species of Botryosphaeriaceae involved in grapevine dieback in Australia. From N. luteum, 18 and 20 were also isolated [78].
Sardiniella urbana, a pathogen of European hackberry trees in Italy, was investigated for its ability to produce secondary metabolites. It produced 1, 18 and 20, which were identified by spectroscopic methods. These compounds were assayed for their phytotoxic, antifungal and zootoxic activities, and among them, only (R)-(-)-mellein was found to be active. In particular, 1 displayed from significant to weak activity towards all plant pathogens tested at 0.2 mg/plug. Athelia rolfsii, Botrytis cinerea and Sclerotinia sclerotiorum were the most sensitive species. On the contrary, Alternaria brassicicola, Fusarium graminearum and Phytophthora cambivora were less sensitive. In the Artemia salina bioassay, 1 caused 100% larval mortality at 200 μg/mL. The LC50 value was 102 μg/mL after 36 h of exposure to the metabolite [79].
Aspergillus flocculus, an endophyte isolated from the stem of the medicinal plant Markhamia platycalyx, was investigated for its ability to synthesize bioactive anticancer and anti-trypanosome secondary metabolites. From the fermentation culture of the fungus were isolated several metabolites belonging to different classes of natural compounds. Among them were isolated some mellein derivatives identified as 1, 18, 19, 20, 31, 34 and botryoisocoumarin A (49, Figure 3). Compounds 18, 34, 49 and 1 inhibited the growth of chronic myelogenous leukemia cell line K562 at 30 μM. Compound 31 exhibited an inhibition of 56% to the sleeping-sickness-causing parasite Trypanosoma brucei brucei [80]. Compound 49, characterized as (3R)-3-methoxymellein, was also previously obtained from the fermentation culture of Botryosphaeria sp. F00741, isolated from the plant epidermis of Avicennia marina [81].
Compound 1 was also recently isolated from the endophytic fungus Colletotrichum gloeosporioides GT-7, obtained from Uncaria rhynchophylla [82].
Xylaria sp. SWUF09-62, a Basidiomycota fungus belonging to the Xylariaceae family, was investigated to explore its ability to produce natural products with anti-inflammation and anti-proliferation activities [83]. This research led to the isolation of several melleins derivatives which were identified by spectroscopic methods as (3S)-7-methoxymellein (50, Figure 3) and (3S)-5,7-dihydroxymellein (51, Figure 3), and their ACs were determined by ECD experiments. (3S)-8-methoxymellein (52, Figure 3), previously synthesized by Kerti et al. [84], was isolated for the first time as a natural compound. In addition, (S)-(+)-mellein (2), (3R)-5-methoxycarbonylmellein (13), (3R,4R)-4-hydroxymellein (18), (3R,4S)-4-hydroxymellein (20) and (3S)-7-hydroxymellein (25), were also isolated. Anti-inflammatory activity screening was carried out by measuring the reduction of NO production in LPS-induced RAW264.7 macrophage cells, and the mellein derivatives showed different degrees of activity. Compound 51 exhibited anti-inflammatory properties by reducing nitric oxide production in LPS-stimulated RAW264.7 cells, indicating possible chemo-preventative and chemo-therapeutic properties [83].
The study conducted by Inose et al. [85] demonstrated the potential of density functional theory (DFT)-based calculations and ECD spectral calculations for structural elucidation of natural compounds. The extract of Periconia macrospinosa KT3863 was studied for secondary metabolite production and two new chlorinated melleins, (3R)-5-chloro-4-hydroxy-6-methoxymellein and (3R)-7-chloro-6-methoxy-8-methoxymellein (53 and 54, Figure 3), were isolated. Furthermore, the authors reported for the first time the complete characterization of the physical properties of the previously isolated (3R)-5-chloro-6-methoxymellein (55, Figure 3) [86]. The results of (DFT)-based calculations were used to estimate the values of the 13C chemical shifts and the spin coupling constants and compare them with experimental data collected by HMBC experiment. The calculations allowed them to determine the relative configurations of 53. In addition, the ACs of 53–55 were established by comparing the experimental ECD spectra with those obtained by time-dependent DFT calculations. The data showed that 53 afforded an ECD spectrum that was almost the mirror image of that of 54. Finally, the authors studied the antifungal activities of 53–55 with Cochliobolus miyabeanus as the model organism; unfortunately no significant inhibition was observed [85].
2.2. Melleins from Plants
The first mellein derivative from a plant was (3R)-6-methoxymellein (3) isolated from bitter carrots (Daucus carota) in 1960 [87]. Successively, compound 3 was isolated in higher yields from carrot root tissue (D. carota) inoculated with Ceratocystis cimbriata, Ceratocystis ulmi, Helminthosporum carbonum or Fusarium oxysporum [88]. After this investigation, it was hypothesized that the production of 3 could resulted from an alteration of the normal metabolism of the plant induced by the presence of fungi together with environmental condition [88]. This proposal was elaborated in a 1963 review, and 6-methoxymellein (3) was classified as a phytoalexin [89]. Thus, 6-methoxymellein (3) plays an important role on the active defenses of whole cold-stored carrots and this property was further investigated [90]. The ethanolic extract of cold-stored carrots slices was purified by TLC and the compounds identified by spectroscopic methods. The isolated compounds were assessed against spore suspensions of Botrytis cinerea. In the spore germination bioassay, the most active inhibitor effect was induced by 6-methoxymellein (3) [90].
Recently, ten different secondary metabolites were isolated from the methanol extract of the twigs and leaves of Garcinia bancana, and their structures were elucidated by spectroscopic methods. Among them (R)-(-)-mellein (1) was identified [91]. In the same year, compound 1 was isolated from the organic extract the stems of Ficus formosana (Moraceae) [92]. Compound 1 was also isolated from the extract of Enicosanthum membranifolium together with clerodermic acid and salicifoline, whose identities were confirmed by using X-ray diffractometric analysis [93]. The extract of the wood of Millettia leucantha proved to contain (R)-(-)-mellein (1) and other secondary metabolites, and the structures of these compounds were assigned by the analysis of their spectroscopic data [94].
Chemical constituents of the whole herb extract of Rhodiola kirilowii Maxim were purified and identified using 1D and 2D NMR methods. Eleven compounds were obtained, and one of them was identified as (R)-(-)-mellein (1) and isolated for the first time from Rhodiola genus [95].
Compound 1 was also isolated from the roots of Antidesma acidum, as identified by spectroscopic methods [96]. (R)-(-)-mellein (1) was also isolated from the roots of another plant Microcos tomentosa [97]. In the same year, from the extract of leaves and stems of Stevia lucida Lagasca, different compounds were isolated, and among them, 1 was identified on the basis of its spectroscopic properties. This was the first report of 1, and in general of an isocoumarin, in Stevia genus [98].
6-Methoxymellein (3), three undescribed and two known xanthones and three biflavanoids were isolated from the methanolic extract of the twigs of Garcinia xanthochymus. Their structures were identified by spectroscopic data; unfortunately the amount of 3 was too low to conduct any bioassay [99].
Masatoshi and co-authors studied the attractiveness of several wood odors to beetles. The beetles were highly attracted to all wood odors of Castanea crenata, Magnolia obovata, Paulownia tomentosa, Prunus jamasakura and Zelkova serrata. The Z. serrata supercritical CO2 extract was the most attractive extract and was analyzed by GC-MS. The major compound detected in the extract was (R)-(-)-mellein (1) and proved to attract the beetles [100]. In the same year, a new bianthraquinone, named by morindaquinone, together with another 12 known secondary metabolites, was isolated from the roots of Morinda coreia. Among those, 1 was isolated and identified according to its spectroscopic data [101].
2.3. Melleins from Insects
The swarming of the carpenter ant, Camponotus herculeanus, is influenced by climatic factors, such as season, temperature and time of day. The synchronization of this swarming is controlled by volatile compounds secreted from the mandibular glands of the males [102]. Brand and his colleagues (1973) analyzed, by GC-MS, the major volatiles in the mandibular gland secretions of C. herculeanus, Camponotus ligniperda and Camponotus pennsylvanicus. The analysis showed that the secretions were dominated by two substances, (R)-(-)-mellein (1) and methyl 6-methylsalicylate, which were further characterized also by NMR data [103]. Another chemical-ecology study on a different species of ant (Rhytidoponera metallica workers) was carried out by Brophy and coauthors in 1981. Thirteen volatile constituents have been characterized in an Australian representative of the primitive ant subfamily Ponerinae, called R. metallica by GC-MS spectrometry. The major component of the total extracts from the bodies of R. metallica workers was (R)-(-)-mellein (1). However, its glandular origin in the gasters of R. metallica is unknown [104].
The production of 1 by ant Camponotus vagus was studied in view of its possible chemotaxonomic and functional significance d [105]. (R)-(-)-mellein (1) was detected as a mandibular gland product of workers of two Camponotus species, and it was also detected in both females and males. Furthermore, compound 1 was also isolated from another formicine species, Polyrhachis doddi. These results highlighted that (R)-(-)-mellein (1) is a characteristic compound of the chemical ecology of ants and is clearly a part of an ant’s defensive exudate [105].
More recently, the volatile components of whole-body extracts of four species of neotropical ants in the formicine genus, as Camponotu kaura, Camponotum sexguttatus, Camponotu ramulorum and Camponotu planatus, were investigated. Volatile mandibular gland compounds were found only in male extracts in three of the species. The results were different within the species; in particular, (R)-(-)-mellein (1) was found only in traces in the C. ramulorum species. In addition, the significance of the mandibular gland secretion for formicid systematics was also discussed [106]. In another study, six compounds were identified in the heads of Camponotus irritibilis; among them (R)-(-)-mellein (1) and 4-hydroxymellein were isolated and identified. Unfortunately, the authors of the study did not assign the absolute configuration to the latter compound. The possibility of semiochemical thrift for these mandibular gland compounds was reviewed and compared with existing data on mandibular gland compounds of other ants of this group [107].
Male wing gland secretion and volatiles emanated from Aphomia sociella (Bee moth) were studied [108]. Two-dimensional gas chromatography-mass spectrometry (GCxGC-TOF-MS), gas chromatography-infrared spectroscopy (GC-FTIR), enantioselective gas chromatography (Es-GC), electroantennography (EAG), gas chromatography with electroantennographic detection (GC-EAD) and NMR were used. The GC-EAD analysis of the male wing gland secretion showed seven different compounds, and among them, (R)-(-)-mellein (1) was identified. Compound 1 and the nona-2,6-dien-4-olide were the most abundant compounds. One or more of these compounds might constitute sex pheromones [108].
 From detailed investigations of genes, amino acid sequences and mechanistic analogies of the enzymes, were possible to identified three general types of PKS: (i) type I, which are very big multifunctional proteins with a single domain. Furthermore, they can also be divided into iterative and non-iterative enzymes; (ii) type II, composed by complex, single, monofunctional proteins; (iii) type III, which differ from the other two by being homodimeric proteins that use a single active site to perform the series of decarboxylation, condensation, cyclization and aromatization reactions. PKS type III are found in plants, bacteria and fungi, PKS type I are typical of bacteria and fungi, while type II are limited to bacteria. Aromatic polyketides, such as melleins, are typical products of PKS type II or type III, although there are some examples of PKS type I capable of producing aromatic rings [110109]. As reviewed in the previous section melleins are mainly produced by pathogenic fungi. Melleins, and more in general polyketides, play a wide range of roles: host-pathogen interaction, facilitations of the host colonization, phytotoxicity [112111]. Almost all fungal PKS currently known are type I systems, while some fungi also possess type III PKS [113112]. Nevertheless, the fungal type I PKS differs from bacterial type I in being iterative [110109][113112]. Fungal PKS have different domains: (i) no reductive PKS (nrPKS) with no reductive steps during chain construction, (ii) partially reducing PKS (prPKS), that usually catalyzes only one reduction during chain extension and (iii) highly reducing PKS (hrPKS) where the level of reduction is varied and clearly subject to a high level of genes expression control [113112].
Fungi usually have 20–50 secondary metabolites genes and their production is highly regulated often in response to specific biotic factors and environmental perturbations. Modern genomic and transcriptomic tools can be used, for pathogenic fungi, to probe the expression of secondary metabolites gene clusters at various stages of infection [113][114][115]. Unfortunately, the absence of whole genome sequences slows down the identification of these target genes.
Focusing our attention on genes sequences and characterized PKS enzymes involved in melleins production in fungi, very little it is available in literature so far.
Saccharopolyspora erythraea, an actinomycete that produces a polyketide with antibiotic activity named erythromycin A was studied [116115]. The modular PKS appointed for the biosynthesis of erythromycin A was studied as model for polyketide synthesis. The genome of S. erythraea revealing a dozen of PKS genes. One of the uncharacterized PKS genes was SACE5532, which encodes a single-module PKS that have sequence homology with several fungal and bacterial type I prPKSs for aromatic polyketide biosynthesis. The product of SACE5532 was identified as (R)-(-)-mellein (1), and the different domains of this prPKS were studied and characterized. The experimental results confirmed the polyketide origin of 1 and might ease the identification of the biosynthetic genes for other dihydroisocoumarins [116115].
More recently, the sequence of fungal PKs involved in (R)-(-)-mellein (1) synthesis in wheat pathogenic fungus Parastagonospora nodorum was reported and the gene, involved in the production of 1 by the wheat pathogen P. nodorum, was studied [117116]. The results showed that SN477 was the most highly expressed PKs gene in planta, and analysis of the DNA sequence indicated that it codes for typical prPKS and was similar with an identical domain architecture to the prPKS ATX from Aspergillus terreus, which synthesizes 6-MSA. These results were confirmed by heterologous expression of SN477 in yeast. The gene knock-out SN477 resulted in a P. nodorum mutant that was not capable of producing (R)-mellein as shown by HPLC metabolic profiling. Thus, SN477 is the first fungal prPKS producing a PKs compound except 6-MSA. However, its biosynthesis was highly parallel to that of 6-MSA but needed additional chain elongation and keto reduction steps [117116].
From detailed investigations of genes, amino acid sequences and mechanistic analogies of the enzymes, were possible to identified three general types of PKS: (i) type I, which are very big multifunctional proteins with a single domain. Furthermore, they can also be divided into iterative and non-iterative enzymes; (ii) type II, composed by complex, single, monofunctional proteins; (iii) type III, which differ from the other two by being homodimeric proteins that use a single active site to perform the series of decarboxylation, condensation, cyclization and aromatization reactions. PKS type III are found in plants, bacteria and fungi, PKS type I are typical of bacteria and fungi, while type II are limited to bacteria. Aromatic polyketides, such as melleins, are typical products of PKS type II or type III, although there are some examples of PKS type I capable of producing aromatic rings [110109]. As reviewed in the previous section melleins are mainly produced by pathogenic fungi. Melleins, and more in general polyketides, play a wide range of roles: host-pathogen interaction, facilitations of the host colonization, phytotoxicity [112111]. Almost all fungal PKS currently known are type I systems, while some fungi also possess type III PKS [113112]. Nevertheless, the fungal type I PKS differs from bacterial type I in being iterative [110109][113112]. Fungal PKS have different domains: (i) no reductive PKS (nrPKS) with no reductive steps during chain construction, (ii) partially reducing PKS (prPKS), that usually catalyzes only one reduction during chain extension and (iii) highly reducing PKS (hrPKS) where the level of reduction is varied and clearly subject to a high level of genes expression control [113112].
Fungi usually have 20–50 secondary metabolites genes and their production is highly regulated often in response to specific biotic factors and environmental perturbations. Modern genomic and transcriptomic tools can be used, for pathogenic fungi, to probe the expression of secondary metabolites gene clusters at various stages of infection [113][114][115]. Unfortunately, the absence of whole genome sequences slows down the identification of these target genes.
Focusing our attention on genes sequences and characterized PKS enzymes involved in melleins production in fungi, very little it is available in literature so far.
Saccharopolyspora erythraea, an actinomycete that produces a polyketide with antibiotic activity named erythromycin A was studied [116115]. The modular PKS appointed for the biosynthesis of erythromycin A was studied as model for polyketide synthesis. The genome of S. erythraea revealing a dozen of PKS genes. One of the uncharacterized PKS genes was SACE5532, which encodes a single-module PKS that have sequence homology with several fungal and bacterial type I prPKSs for aromatic polyketide biosynthesis. The product of SACE5532 was identified as (R)-(-)-mellein (1), and the different domains of this prPKS were studied and characterized. The experimental results confirmed the polyketide origin of 1 and might ease the identification of the biosynthetic genes for other dihydroisocoumarins [116115].
More recently, the sequence of fungal PKs involved in (R)-(-)-mellein (1) synthesis in wheat pathogenic fungus Parastagonospora nodorum was reported and the gene, involved in the production of 1 by the wheat pathogen P. nodorum, was studied [117116]. The results showed that SN477 was the most highly expressed PKs gene in planta, and analysis of the DNA sequence indicated that it codes for typical prPKS and was similar with an identical domain architecture to the prPKS ATX from Aspergillus terreus, which synthesizes 6-MSA. These results were confirmed by heterologous expression of SN477 in yeast. The gene knock-out SN477 resulted in a P. nodorum mutant that was not capable of producing (R)-mellein as shown by HPLC metabolic profiling. Thus, SN477 is the first fungal prPKS producing a PKs compound except 6-MSA. However, its biosynthesis was highly parallel to that of 6-MSA but needed additional chain elongation and keto reduction steps [117116].
2.4. Melleins from Bacteria
Only one article on (R)-(-)-mellein (1) produced by bacteria is available. Volatile compounds released by 50 bacterial strains have been collected, and the obtained headspace extracts were analyzed by GC-MS, which is a fundamental tool for the discovery of natural compounds that might be missed by using traditional techniques [109]. Furthermore, Saccharopolyspora erythraea was found to produce compound 1 for the first time. Aside from (R)-(-)-mellein (1), other insect pheromones such as methyl 6-methylsalicylate, methyl 6-ethylsalicylate, pyrrole-2-carboxylate, conophthorin and chalcogran were produced by bacteria strains. Considering the symbiotic relationships between actinomycetes and insects, further investigations should be performed on the origins of these compounds in these species [109].3. Biosynthetic Pathways and Gene Involved in Mellein Production
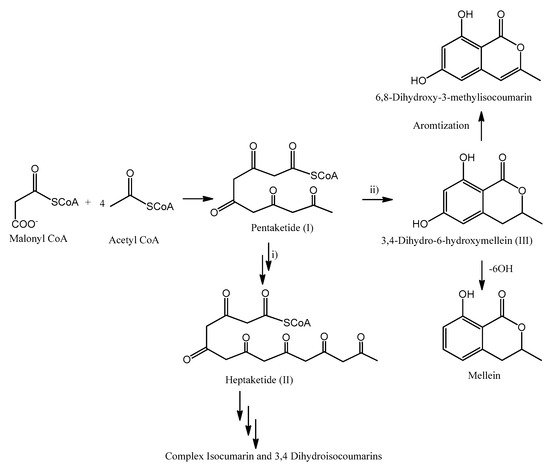
Isocoumarins, 3,4-dihydroisocoumarins and melleins belong to the class of secondary metabolites named polyketides. Considering their activities and their biological roles, this class of natural compounds is one of the major secondary metabolite classes. In general, they occur in fungi, plants, bacteria and marine organisms. Isocoumarines, 3,4-dihydroisocoumarins and melleins show a common biosynthetic origin: they are related to the fatty acid biosynthesis, which reactions are catalyzed by enzymes named polyketides synthase (PKS) [110109]. The possible sequence of reactions involved in their biosynthesis is outlined in Figure 4. Starting form malonyl-CoA and successive Claisen condensation with 4 acetyl-CoA moieties, pentaketide (I) is generated. This reaction initially produces a β−chetoester, and then the ketonic group is reduced after each stage of condensation and before the subsequent phase of chain elongation [110109]. Pentaketide (I) might be involved in different reactions: (i) Further chain elongation, spawning the heptaketide (II). Post-PKS modification of II may result in a variety of more complex isocoumarines or 3,4-dihydroisocoumarins. (ii) Cyclization reaction, which produces the typical six-membered lactone ring synthetizing 6-hydroxymellein (III). Further modification of III my include aromatization, generating 6,8-dihydroxy-3-methylisocoumarin, or 6-OH dehydration, forming mellein [109][110][111].
Figure 4. Possible reactions involved with isocoumarines, 3,4-dihydroisocoumarins and melleins biosyntheses.
Social insects have developed strong antimicrobial defenses against infection of pathogens and parasites. Indeed, antimicrobial compounds have been identified in Reticulitermes speratus (Kolbe) organic extracts. Mitaka and his colleagues (2019) identified (R)-(-)-mellein (1) using GC-MS analysis. Antifungal assays showed that compound 1 has an inhibitory effect on the growth of Metarhizium anisopliae and Beauveria bassiana. These results suggest that R. speratus use (R)-(-)-mellein (1) to fight the pathogenic fungi; unfortunately the termite-egg-mimicking fungus has resistance against 1 [117].
2.4. Melleins from Bacteria
Only one article on (R)-(-)-mellein (1) produced by bacteria is available. Volatile compounds released by 50 bacterial strains have been collected, and the obtained headspace extracts were analyzed by GC-MS, which is a fundamental tool for the discovery of natural compounds that might be missed by using traditional techniques [118].
Furthermore, Saccharopolyspora erythraea was found to produce compound 1 for the first time. Aside from (R)-(-)-mellein (1), other insect pheromones such as methyl 6-methylsalicylate, methyl 6-ethylsalicylate, pyrrole-2-carboxylate, conophthorin and chalcogran were produced by bacteria strains. Considering the symbiotic relationships between actinomycetes and insects, further investigations should be performed on the origins of these compounds in these species [118].
References
- Valio, I.F.M. Effect of Endogenous coumarin on the germination of seeds of Coumarouna odorta Aublet. J. Exp. Bot. 1973, 24, 442–449.
- Barry, R.D. Isocoumarins. Developments since 1950. Chem. Rev. 1964, 64, 229–260.
- Nishikawa, H. Biochemistry of filamentous fungi. II: A metabolic product of Aspergillus melleus Yukawa. Part I and Part II. J. Agric. Chem. Soc. Jpn. 1933, 9, 107–109, 148–151.
- Zepnik, H.; Pähler, A.; Schauer, U.; Dekant, W. Ochratoxin A-induced tumor formation: Is there a role of reactive ochratoxin A metabolites? Toxicol. Sci. 2001, 59, 59–67.
- Braca, A.; Bader, A.; De Tommasi, N. Plant and fungi 3,4-dihydroisocoumarins. Bioact. Nat. Prod. 2012, 37, 191–215.
- Saeed, A. Isocoumarins, miraculous natural products blessed with diverse pharmacological activities. Eur. J. Med. Chem. 2016, 116, 290–317.
- Saeed, J.S. Fungal volatiles–a survey from edible mushrooms to moulds. Nat. Prod. Rep. 2017, 34, 310–328.
- Pal, S.; Chatare, V.; Pal, M. Isocoumarin and its derivatives: An overview on their synthesis and applications. Curr. Org. Chem. 2011, 15, 782–800.
- Saddiqa, A.; Usman, M.; Çakmak, O. Isocoumarins and 3,4-dihydroisocoumarins, amazing natural products: A review. Turk. J. Chem. 2017, 41, 153–178.
- Ortiz, A.; Castro, M.; Sansinenea, E. 3,4-Dihydroisocoumarins, interesting natural products: Isolation, organic syntheses and biological activities. Curr. Org. Synth. 2019, 16, 112–129.
- Blair, J.; Newbold, G.T. Lactones. Part II. The structure of mellein. J. Chem. Soc. 1955, 2871.
- Arakawa, H. Absolute configuration of mullein. Bull. Chem. Soc. Jpn. 1968, 41, 2541.
- Arakawa, H.; Torimoto, N.; Masui, Y. Absolute configuration of optically active, naturally occurring dihydroisocoumarins. II. Determination of the absolute configuration of agrimonolide and mellein. Liebigs Ann. Chem. 1969, 728, 152–157.
- Patterson, E.L.; Andres, W.W.; Bohonos, N. Isolation of the optical antipode of mellein from an unidentified fungus. Cell. Mol. Life Sci. 1966, 22, 209–210.
- Grove, J.F.; Pople, M. Metabolic products of Fusarium larvarum fuckel. The fusarentins and the absolute configuration of monocerin. J. Chem. Soc. Perkin Trans. 1979, 1, 2048–2051.
- Aue, R.; Mauli, R.; Sigg, H.P. Production of 6-methoxy-mellein by Sporormia bipartis Cain. Cell. Mol. Life Sci. 1966, 22, 575.
- Ballio, A.; Barcellona, S.; Santurbano, B. 5-Methylmellein, a new natural dihydroisocoumarin. Tetrahedron Lett. 1966, 7, 3723–3726.
- McGahren, W.J.; Mitscher, L.A. Dihydroisocoumarins from a Sporormia fungus. J. Org. Chem. 1968, 33, 1577–1580.
- Sasaki, M.; Kaneko, Y.; Oshita, K.; Takamatsu, H.; Asao, Y.; Yokotsuka, T. Studies on the compounds produced by molds. Agric. Biol. Chem. 1970, 34, 1296–1300.
- Aldridge, D.C.; Galt, S.; Giles, D.; Turner, W.B. Metabolites of Lasiodiplodia theobromae. J. Chem. Soc. C 1971, 1623.
- Cole, R.J.; Moore, J.H.; Davis, N.D.; Kirksey, J.W.; Diener, U.L. 4-Hydroxymellein. New metabolite of Aspergillus ochraceus. J. Agric. Food Chem. 1971, 19, 909–911.
- Moore, J.H.; Davis, N.D.; Diener, U.L. Mellein and 4-Hydroxymellein production by Aspergillus ochraceus Wilhelm. Appl. Microbiol. 1972, 23, 1067–1072.
- Camarda, L.; Merlini, L.; Nasini, G. Metabolites of Cercospora. Taiwapyrone, an α-pyrone of unusual structure from Cercospora taiwanensis. Phytochemistry 1976, 15, 537–539.
- Assante, G.; Locci, R.; Camarda, L.; Merlini, L.; Nasini, G. Screening of the genus Cercospora for secondary metabolites. Phytochemistry 1977, 16, 243–247.
- Devys, M.; Bousquet, J.F.; Kollmann, A.; Barbier, M. Dihydroisocoumarins and mycophenolic acid of the culture medium of the plant pathogenic fungus Septoria nodorum. Phytochemistry 1980, 19, 2221–2222.
- Anderson, J.R.; Edwards, R.L.; Whalley, A.J. Metabolites of the higher fungi. Part 21. 3-Methyl-3, 4-dihydroisocoumarins and related compounds from the ascomycete family Xylariaceae. J. Chem. Soc. Perkin Trans. 1 1983, 2185–2192.
- Claydon, N.; Grove, J.F.; Pople, M. Elm bark beetle boring and feeding deterrents from Phomopsis oblonga. Phytochemistry 1985, 24, 937–943.
- Okuno, T.; Oikawa, S.; Goto, T.; Sawai, K.; Shirahama, H.; Matsumoto, T. Structures and phytotoxicity of metabolites from Valsa ceratosperma. Agric. Biol. Chem. 1986, 50, 997–1001.
- Ayer, W.A.; Shewchuk, L.M. Metabolites of Nectria fuckeliana. J. Nat. Prod. 1986, 49, 947–948.
- Venkatasubbaiah, P.; Chilton, W.S. Phytotoxins of Botryosphaeria obtusa. J. Nat. Prod. 1990, 53, 1628–1630.
- Venkatasubbaiah, P. Effect of phytotoxins produced by Botryosphaeria obtusa, the cause of black rot of apple fruit and frogeye leaf spot. Phytopathology 1991, 81, 243.
- Venkatasubbaiah, P.; Baudoin, A.B.A.M.; Chilton, W.S. Leaf spot of hemp dogbane caused by Stagonospora apocyni, and its phytotoxins. J. Phytopathol. 1992, 135, 309–316.
- Sachse, J. Identification and determination of mellein in cultures of the fungus Septoria nodorum (Berk.) by thin-layer and high-performance liquid chromatography. J. Chromatogr. A 1992, 609, 349–353.
- Devys, M.; Barbier, M.; Bousquet, J.-F.; Kollmann, A. Notes: Isolation of the new (-)-(3R,4S)-4-hydroxymellein from the fungus Septoria nodorum Berk. Z. Naturforsch. C 1992, 47, 779–781.
- Parisi, A.; Piattelli, M.; Tringali, C.; Lio, G.M.D.S. Identification of the phytotoxin mellein in culture fluids of Phoma tracheiphila. Phytochemistry 1993, 32, 865–867.
- Schulz, B.; Sucker, J.; Aust, H.; Krohn, K.; Ludewig, K.; Jones, P.; Döring, D. Biologically active secondary metabolites of endophytic Pezicula species. Mycol. Res. 1995, 99, 1007–1015.
- Höller, U.; König, G.M.; Wright, A.D. Three new metabolites from marine-derived fungi of the genera Coniothyrium and Microsphaeropsis. J. Nat. Prod. 1999, 62, 114–118.
- Dai, J.-R.; Carte, B.K.; Sidebottom, P.J.; Yew, A.L.S.; Ng, S.-B.; Huang, Y.; Butler, M.S. Circumdatin G, a new alkaloid from the fungus Aspergillus ochraceus. J. Nat. Prod. 2001, 64, 125–126.
- Kokubun, T.; Veitch, N.C.; Bridge, P.D.; Simmonds, M.S.J. Dihydroisocoumarins and a tetralone from Cytospora eucalypticola. Phytochemistry 2003, 62, 779–782.
- Klemke, C.; Kehraus, S.; Wright, A.D.; König, G.M. New Secondary metabolites from the marine endophytic fungus Apiospora montagnei. J. Nat. Prod. 2004, 67, 1058–1063.
- Cabras, A.; Mannoni, M.A.; Serra, S.; Andolfi, A.; Fiore, M.; Evidente, A. Occurrence, isolation and biological activity of phytotoxic metabolites produced in vitro by Sphaeropsis sapinea, pathogenic fungus of Pinus radiata. Eur. J. Plant. Pathol. 2006, 115, 187–193.
- Pongcharoen, W.; Rukachaisirikul, V.; Phongpaichit, S.; Sakayaroj, J. A new dihydrobenzofuran derivative from the endophytic fungus Botryosphaeria mamane PSU-M76. Chem. Pharm. Bull. 2007, 55, 1404–1405.
- Sumarah, M.W.; Puniani, E.; Blackwell, B.A.; Miller, J.D. Characterization of polyketide metabolites from foliar endophytes of Picea glauca. J. Nat. Prod. 2008, 71, 1393–1398.
- Djoukeng, J.D.; Polli, S.; Larignon, P.; Mansour, E.A. Identification of phytotoxins from Botryosphaeria obtusa, a pathogen of black dead arm disease of grapevine. Eur. J. Plant. Pathol. 2009, 124, 303–308.
- Rukachaisirikul, V.; Arunpanichlert, J.; Sukpondma, Y.; Phongpaichit, S.; Sakayaroj, J. Metabolites from the endophytic fungi Botryosphaeria rhodina PSU-M35 and PPSU-M114. Tetrahedron 2009, 65, 10590–10595.
- Li, Y.; Lu, C.; Hu, Z.; Huang, Y.; Shen, Y.-M. Secondary metabolites of Tubercularia sp. TF5, an endophytic fungal strain of Taxus mairei. Nat. Prod. Res. 2009, 23, 70–76.
- Evidente, A.; Punzo, B.; Andolfi, A.; Cimmino, A.; Melck, D.; Luque, J. Lipophilic phytotoxins produced by Neofusicoccum parvum, a grapevine canker agent. Phytopathol. Mediterr. 2010, 49, 74–79.
- Evidente, A.; Masi, M.; Linaldeddu, B.T.; Franceschini, A.; Scanu, B.; Cimmino, A.; Andolfi, A.; Motta, A.; Maddau, L. Afritoxinones A and B, dihydrofuropyran-2-ones produced by Diplodia africana the causal agent of branch dieback on Juniperus phoenicea. Phytochemistry 2012, 77, 245–250.
- Cheng, M.-J.; Wu, M.-D.; Hsieh, S.-Y.; Chen, I.-S.; Yuan, G.-F. Secondary metabolites isolated from the fungus Biscogniauxia cylindrospora BCRC 33717. Chem. Nat. Compd. 2011, 47, 527–530.
- Cheng, M.-J.; Cheng, M.-J.; Yuan, G.-F.; Chen, Y.-L.; Su, Y.-S.; Hsieh, M.-T.; Chen, I.-S. Secondary metabolites and cytotoxic activities from the endophytic fungus Annulohypoxylon squamulosum. Phytochem. Lett. 2012, 5, 219–223.
- Araújo, F.D.D.S.; Fávaro, L.C.D.L.; Araújo, W.L.; De Oliveira, F.L.; Aparicio, R.; Marsaioli, A.J. Epicolactone—Natural product isolated from the sugarcane endophytic fungus Epicoccum nigrum. Eur. J. Org. Chem. 2012, 2012, 5225–5230.
- Amand, S.; Langenfeld, A.; Blond, A.; Dupont, J.; Nay, B.; Prado, S. Guaiane sesquiterpenes from Biscogniauxia nummularia featuring potent antigerminative activity. J. Nat. Prod. 2012, 75, 798–801.
- Hussain, H.; Krohn, K.; Schulz, B.; Draeger, S.; Nazir, M.; Saleem, M. Two new antimicrobial metabolites from the endophytic fungus, Seimatosporium sp. Nat. Prod. Commun. 2012, 7, 1934578–1200700305.
- Klaiklay, S.; Rukachaisirikul, V.; Sukpondma, Y.; Phongpaichit, S.; Buatong, J.; Bussaban, B. Metabolites from the mangrove-derived fungus Xylaria cubensis PSU-MA34. Arch. Pharm. Res. 2012, 35, 1127–1131.
- Montenegro, T.G.C.; Rodrigues, F.A.R.; Jimenez, P.C.; Angelim, A.L.; Melo, V.M.M.; Filho, E.R.; Oliveira, M.C.F.; Costa-Lotufo, L.V. Cytotoxic activity of fungal strains isolated from the ascidian Eudistoma vannamei. Chem. Biodivers. 2012, 9, 2203–2209.
- Zhao, J.H.; Zhang, Y.; Wang, L.W.; Wang, J.Y.; Zhang, C.L. Bioactive secondary metabolites from Nigrospora sp. LLGLM003, an endophytic fungus of the medicinal plant Moringa oleifera Lam. World J. Microbiol. Biotechnol. 2012, 28, 2107–2112.
- Kendagor, A.C.; Langat, M.K.; Cheplogoi, P.K.; Omolo, J.O. Larvicidal activity of mellein from cultures of an ascomycete Pezicula livida against Aedes aegypti. Int. J. Life Sci. Biotechnol. Pharm. Res. 2013, 2, 70–80.
- Yun, K.; Feng, Z.; Choi, H.D.; Kang, J.S.; Son, B.W. New production of (R)-(–)-5-bromomellein, a dihydroisocoumarin derivative from the marine-derived fungus Aspergillus ochraceus. Chem. Nat. Compd. 2013, 49, 24–26.
- Ramos, H.P.; Simão, M.R.; De Souza, J.M.; Magalhães, L.G.; Rodrigues, V.; Ambrósio, S.R.; Said, S. Evaluation of dihydroisocoumarins produced by the endophytic fungus Arthrinium state of Apiospora montagnei against Schistosoma mansoni. Nat. Prod. Res. 2013, 27, 2240–2243.
- Rukachaisirikul, V.; Buadam, S.; Sukpondma, Y.; Phongpaichit, S.; Sakayaroj, J.; Hutadilok-Towatana, N. Indanone and mellein derivatives from the Garcinia-derived fungus Xylaria sp. PSU-G12. Phytochem. Lett. 2013, 6, 135–138.
- Hussain, H.; Jabeen, F.; Krohn, K.; Al-Harrasi, A.; Ahmad, M.; Mabood, F.; Shah, A.; Badshah, A.; Rehman, N.U.; Green, I.R.; et al. Antimicrobial activity of two mellein derivatives isolated from an endophytic fungus. Med. Chem. Res. 2014, 24, 2111–2114.
- Ibrahim, A.; Sørensen, D.; Jenkins, H.A.; McCarry, B.E.; Sumarah, M.W. New diplosporin and agistatine derivatives produced by the fungal endophyte Xylaria sp. isolated from Vitis labrusca. Phytochem. Lett. 2014, 9, 179–183.
- Qian, C.; Fu, Y.-H.; Jiang, F.; Xu, Z.-H.; Cheng, D.; Ding, B.; Gao, C.-X.; Ding, Z. Lasiodiplodia sp. ME4-2, an endophytic fungus from the floral parts of Viscum coloratum, produces indole-3-carboxylic acid and other aromatic metabolites. BMC Microbiol. 2014, 14, 297.
- Wang, J.; Wang, G.; Zhang, Y.; Zheng, B.; Zhang, C.; Wang, L. Isolation and identification of an endophytic fungus Pezicula sp. in Forsythia viridissima and its secondary metabolites. World J. Microbiol. Biotechnol. 2014, 30, 2639–2644.
- Sorres, J.; Nirma, C.; Touré, S.; Eparvier, V.; Stien, D. Two new isopimarane diterpenoids from the endophytic fungus Xylaria sp. SNB-GTC2501. Tetrahedron Lett. 2015, 56, 4596–4598.
- Prabpai, S.; Wiyakrutta, S.; Sriubolmas, N.; Kongsaeree, P. Antimycobacterial dihydronaphthalenone from the endophytic fungus Nodulisporium sp. of Antidesma ghaesembilla. Phytochem. Lett. 2015, 13, 375–378.
- Ju, Z.; Lin, X.; Lü, X.; Tu, Z.; Wang, J.; Kaliyaperumal, K.; Liu, J.; Tian, Y.; Xu, S.-H.; Liu, Y.-H. Botryoisocoumarin A, a new COX-2 inhibitor from the mangrove Kandelia candel endophytic fungus Botryosphaeria sp. KcF6. J. Antibiot. 2015, 68, 653–656.
- Yang, N.; Ma, Q.; Huang, S.; Dai, H.; Guo, Z.; Lu, X.; Wang, Y.; Yu, Z.; Zhao, Y. Chemical constituents from cultures of the fungus Marasmiellus ramealis (Bull.) Singer. J. Braz. Chem. Soc. 2014, 26, 9–13.
- Mansour, E.A.; Debieux, J.-L.; Ramírez-Suero, M.; Bénard-Gellon, M.; Magnin-Robert, M.; Spagnolo, A.; Chong, J.; Farine, S.; Bertsch, C.; L’Haridon, F.; et al. Phytotoxic metabolites from Neofusicoccum parvum, a pathogen of Botryosphaeria dieback of grapevine. Phytochemistry 2015, 115, 207–215.
- Cimmino, A.; Cinelli, T.; Masi, M.; Reveglia, P.; Da Silva, M.A.; Mugnai, L.; Michereff, S.J.; Surico, G.; Evidente, A. Phytotoxic lipophilic metabolites produced by grapevine strains of Lasiodiplodia species in Brazil. J. Agric. Food Chem. 2017, 65, 1102–1107.
- Kamal, N.; Viegelmann, C.V.; Clements, C.J.; Edrada-Ebel, R. Metabolomics-guided isolation of anti-trypanosomal metabolites from the endophytic fungus Lasiodiplodia theobromae. Planta Med. 2016, 83, 565–573.
- Cimmino, A.; Maddau, L.; Masi, M.; Linaldeddu, B.T.; Pescitelli, G.; Evidente, A. Fraxitoxin, a new isochromanone isolated from Diplodia fraxini. Chem. Biodivers. 2017, 14, e1700325.
- Yang, J.X.; Chen, Y.; Huang, C.; She, Z.; Lin, Y. A new isochroman derivative from the marine fungus Phomopsis sp. (No. ZH-111). Chem. Nat. Compd. 2011, 47, 13–16.
- Zhang, L.-H.; Li, S.-G.; Wu, H.-H.; Chen, G.; Li, L.; Bai, J.; Hua, H.; Wang, H.-F.; Pei, Y.-H. 3,4-Dihydroisocoumarin derivatives from the marine-derived fungus Paraconiothyrium sporulosum YK-03. Phytochem. Lett. 2017, 20, 200–203.
- Zhao, M.; Yuan, L.-Y.; Guo, D.-L.; Ye, Y.; Da-Wa, Z.-M.; Wang, X.-L.; Ma, F.-W.; Chen, L.; Gu, Y.-C.; Ding, L.-S.; et al. Bioactive halogenated dihydroisocoumarins produced by the endophytic fungus Lachnum palmae isolated from Przewalskia tangutica. Phytochemistry 2018, 148, 97–103.
- Shigemori, H.; Tanabe, Y.; Matsumoto, T.; Hosoya, T.; Sato, H. Palmaerins A-D, new chlorinated and brominated dihydroisocoumarins with antimicrobial and plant growth regulating activities from Discomycete Lachnum palmae. Heterocycles 2013, 87, 1481.
- Shigemoto, R.; Matsumoto, T.; Masuo, S.; Takaya, N. 5-Methylmellein is a novel inhibitor of fungal sirtuin and modulates fungal secondary metabolite production. J. Gen. Appl. Microbiol. 2018, 64, 240–247.
- Reveglia, P.; Savocchia, S.; Billones-Baaijens, R.; Masi, M.; Cimmino, A.; Evidente, A. Phytotoxic metabolites by nine species of Botryosphaeriaceae involved in grapevine dieback in Australia and identification of those produced by Diplodia mutila, Diplodia seriata, Neofusicoccum australe and Neofusicoccum luteum. Nat. Prod. Res. 2018, 33, 2223–2229.
- Cimmino, A.; Maddau, L.; Masi, M.; Linaldeddu, B.T.; Evidente, A. Secondary metabolites produced by Sardiniella urbana, a new emerging pathogen on European hackberry. Nat. Prod. Res. 2018, 33, 1–8.
- Tawfike, A.F.; Romli, M.; Clements, C.; Abbott, G.; Young, L.; Schumacher, M.; Diederich, M.; Farag, M.; Edrada-Ebel, R. Isolation of anticancer and anti-trypanosome secondary metabolites from the endophytic fungus Aspergillus flocculus via bioactivity guided isolation and MS based metabolomics. J. Chromatogr. B 2019, 71–83.
- Xu, Y.; Lu, C.; Zheng, Z. A new 3,4-dihydroisocoumarin isolated from Botryosphaeria sp. F00741. Chem. Nat. Compd. 2012, 48, 205–207.
- Yang, Z.-D.; Li, Z.-J.; Zhao, J.-W.; Sun, J.-H.; Yang, L.-J.; Shu, Z.-M. Secondary metabolites and PI3K inhibitory activity of Colletotrichum gloeosporioides, a fungal endophyte of Uncaria rhynchophylla. Curr. Microbiol. 2019, 76, 904–908.
- Patjana, T.; Jantaharn, P.; Katrun, P.; Mongkolthanaruk, W.; Suwannasai, N.; Senawong, T.; Tontapha, S.; Amornkitbumrung, V.; McCloskey, S. Anti-inflammatory and cytotoxic agents from Xylaria sp. SWUF09-62 fungus. Nat. Prod. Res. 2019, 24, 1–10.
- Kerti, G.; Kurtán, T.; Illyés, T.-Z.; Kövér, K.E.; Sólyom, S.; Pescitelli, G.; Fujioka, N.; Berova, N.; Antus, S. Enantioselective synthesis of 3-methylisochromans and determination of their absolute configurations by circular dichroism. Eur. J. Org. Chem. 2007, 2007, 296–305.
- Inose, K.; Tanaka, K.; Koshino, H.; Hashimoto, M. Cyclopericodiol and new chlorinated melleins isolated from Periconia macrospinosa KT3863. Tetrahedron 2019, 75, 130470.
- Krohn, K.; Bahramsari, R.; Flörke, U.; Ludewig, K.; Kliche-Spory, C.; Michel, A.; Aust, H.-J.; Draeger, S.; Schulz, B.; Antus, S. Dihydroisocoumarins from fungi: Isolation, structure elucidation, circular dichroism and biological activity. Phytochemistry 1997, 45, 313–320.
- Condon, P.; Kuc, J. Isolation of a fungitoxic compound from carrot root tissue inoculated with Cerato-cystis fimbriata. Phytopathology 1960, 50, 267–270.
- Condon, P.; Kuc, J.; Draudt, H.N. Production of 3-methyl-6-methoxy-8-hydroxy-3, 4-dihydroisocoumarin by carrot root tissue. Phytopathology 1963, 53, 1244–1250.
- Kuc, J. Resistance of plants to infectious agents. Annu. Rev. Microbiol. 1966, 20, 337–370.
- Harding, V.; Heale, J. Isolation and identification of the antifungal compounds accumulating in the induced resistance response of carrot root slices to Botrytis cinerea. Physiol. Plant. Pathol. 1980, 17, 277–289.
- Rukachaisirikul, V.; Naklue, W.; Sukpondma, Y.; Phongpaichit, S. An antibacterial biphenyl derivative from Garcinia bancana MIQ. Chem. Pharm. Bull. 2005, 53, 342–343.
- Sheu, Y.-W.; Chiang, L.-C.; Chen, Y.-C.; Tsai, I.-L.; Chen, I.-S. Cytotoxic flavonoids and new chromenes from Ficus formosana f. formosana. Planta Medica 2005, 71, 1165–1167.
- Efdi, M.; Itoh, T.; Akao, Y.; Nozawa, Y.; Koketsu, M.; Ishihara, H. The isolation of secondary metabolites and in vitro potent anti-cancer activity of clerodermic acid from Enicosanthum membranifolium. Bioorg. Med. Chem. 2007, 15, 3667–3671.
- Rayanil, K.-O.; Bunchornmaspan, P.; Tuntiwachwuttikul, P. A new phenolic compound with anticancer activity from the wood of Millettia leucantha. Arch. Pharmacal Res. 2011, 34, 881–886.
- Yang, L.-M.; Fu, H.-Z. Chemical constituents of Rhodiola kirilowii Maxim. J. Chin. Pharm. Sci. 2011, 20, 154–158.
- Kaennakam, S.; Sichaem, J.; Siripong, P.; Tip-Pyang, S. A new cytotoxic phenolic derivative from the roots of Antidesma acidum. Nat. Prod. Commun. 2013, 8, 1111–1113.
- Kaennakam, S.; Sichaem, J.; Khumkratok, S.; Siripong, P.; Tip-Pyang, S. A new taraxerol derivative from the roots of Microcos tomentosa. Nat. Prod. Commun. 2013, 8, 1371–1372.
- Chacón-Morales, P.; Amaro-Luis, J.M.; Bahsas, A. Isolation and characterization of (+)-mellein, the first isocoumarin reported in Stevia genus. Avances Química 2013, 8, 145–151.
- Trisuwan, K.; Boonyaketgoson, S.; Rukachaisirikul, V.; Phongpaichit, S. Oxygenated xanthones and biflavanoids from the twigs of Garcinia xanthochymus. Tetrahedron Lett. 2014, 55, 3600–3602.
- Hori, M.; Aoki, Y.; Shinoda, K.; Chiba, M.; Sasaki, R. Wood volatiles as attractants of the confused flour beetle, Tribolium confusum (Coleoptera: Tenebrionidae). Sci. Rep. 2019, 9, 11544–11548.
- Chokchaisiri, S.; Siriwattanasathien, Y.; Thongbamrer, C.; Suksamrarn, A.; Rukachaisirikul, T. Morindaquinone, a new bianthraquinone from Morinda coreia roots. Nat. Prod. Res. 2019, 21, 1–7.
- Bradshaw, J.W.S.; Howse, P.E. Sociochemicals of Ants. In Chemical Ecology of Insects; Springer: Boston, MA, USA, 1984; pp. 429–473.
- Brand, J.; Fales, H.; Sokoloski, E.; MacConnell, J.; Blum, M.; Duffield, R. Identification of mellein in the mandibular gland secretions of carpenter ants. Life Sci. 1973, 13, 201–211.
- Brophy, J.; Cavill, G.; Plant, W. Volatile constituents of an Australian ponerine ant Rhytidoponera metallica. Insect Biochem. 1981, 11, 307–310.
- Blum, M.S.; Morel, L.; Fales, H.M. Chemistry of the mandibular gland secretion of the ant Camponotus vagus. Comp. Biochem. Physiol. Part. B Comp. Biochem. 1987, 86, 251–252.
- Torres, J.A.; Snelling, R.R.; Blum, M.S.; Flournoy, R.C.; Jones, T.H.; Duffield, R.M. Mandibular gland chemistry of four Caribbean species of Camponotus (Hymenoptera: Formicidae). Biochem. Syst. Ecol. 2001, 29, 673–680.
- Voegtle, H.L.; Jones, T.H.; Davidson, D.W.; Snelling, R.R. E-2-Ethylhexenal, E-2-ethyl-2-hexenol, mellein, and 4-hydroxymellein in Camponotus species from Brunei. J. Chem. Ecol. 2008, 34, 215–219.
- Kalinova, B.; Kindl, J.; Jiros, P.; Zacek, P.; Vasickova, S.; Budesinsky, M.; Valterova, I. Composition and electrophysiological activity of constituents identified in male wing gland secretion of the bumblebee parasite Aphomia sociella. J. Nat. Prod. 2009, 72, 8–13.
- Citron, C.A.; Rabe, P.; Dickschat, J.S. The scent of bacteria: Headspace analysis for the discovery of natural products. J. Nat. Prod. 2012, 75, 1765–1776.Dewick, P.M. Medicinal Natural Products: A Biosynthetic Approach, 3rd ed.; John Wiley & Sons: Chicester, UK, 2009; pp. 161–162.
- Dewick, P.M. Medicinal Natural Products: A Biosynthetic Approach, 3rd ed.; John Wiley & Sons: Chicester, UK, 2009; pp. 161–162.Noor, A.; Almasri, D.M.; Bagalagel, A.A.; Abdallah, H.M.; Mohamed, S.G.A.; Mohamed, G.A.; Ibrahim, S.R.M. Naturally occurring isocoumarins derivatives from endophytic fungi: Sources, isolation, structural characterization, biosynthesis, and biological activities. Molecules 2020, 25, 395.
- Noor, A.; Almasri, D.M.; Bagalagel, A.A.; Abdallah, H.M.; Mohamed, S.G.A.; Mohamed, G.A.; Ibrahim, S.R.M. Naturally occurring isocoumarins derivatives from endophytic fungi: Sources, isolation, structural characterization, biosynthesis, and biological activities. Molecules 2020, 25, 395.Mérillon, J.-M.; Ramawat, K.G. Fungal Metabolites; Springer: New York, NY, USA, 2017.
- Mérillon, J.-M.; Ramawat, K.G. Fungal Metabolites; Springer: New York, NY, USA, 2017.Cox, R.J.; Skellam, E.; Williams, K. Biosynthesis of fungal polyketides. Physiol. Genet. 2018, 385–412.
- Cox, R.J.; Skellam, E.; Williams, K. Biosynthesis of fungal polyketides. Physiol. Genet. 2018, 385–412.Yaegashi, J.; Oakley, B.R.; Wang, C.C.C. Recent advances in genome mining of secondary metabolite biosynthetic gene clusters and the development of heterologous expression systems in Aspergillus nidulans. J. Ind. Microbiol. Biotechnol. 2013, 41, 433–442.
- Yaegashi, J.; Oakley, B.R.; Wang, C.C.C. Recent advances in genome mining of secondary metabolite biosynthetic gene clusters and the development of heterologous expression systems in Aspergillus nidulans. J. Ind. Microbiol. Biotechnol. 2013, 41, 433–442.Cacho, R.A.; Tang, Y.; Chooi, Y.-H. Next-generation sequencing approach for connecting secondary metabolites to biosynthetic gene clusters in fungi. Front. Microbiol. 2015, 5, 774.
- Cacho, R.A.; Tang, Y.; Chooi, Y.-H. Next-generation sequencing approach for connecting secondary metabolites to biosynthetic gene clusters in fungi. Front. Microbiol. 2015, 5, 774.Sun, H.; Ho, C.L.; Ding, F.; Soehano, I.; Liu, X.-W.; Liang, Z.-X. Synthesis of (R)-mellein by a partially reducing iterative polyketide synthase. J. Am. Chem. Soc. 2012, 134, 11924–11927.
- Sun, H.; Ho, C.L.; Ding, F.; Soehano, I.; Liu, X.-W.; Liang, Z.-X. Synthesis of (R)-mellein by a partially reducing iterative polyketide synthase. J. Am. Chem. Soc. 2012, 134, 11924–11927.Chooi, Y.-H.; Krill, C.; Barrow, R.A.; Chen, S.; Trengove, R.; Oliver, R.P.; Solomon, P. An in planta-expressed polyketide synthase produces (R)-mellein in the wheat pathogen Parastagonospora nodorum. Appl. Environ. Microbiol. 2014, 81, 177–186.
- Chooi, Y.-H.; Krill, C.; Barrow, R.A.; Chen, S.; Trengove, R.; Oliver, R.P.; Solomon, P. An in planta-expressed polyketide synthase produces (R)-mellein in the wheat pathogen Parastagonospora nodorum. Appl. Environ. Microbiol. 2014, 81, 177–186.Mitaka, Y.; Mori, N.; Matsuura, K. A termite fungistatic compound, mellein, inhibits entomopathogenic fungi but not egg-mimicking termite ball fungi. Appl. Entomol. Zool. 2018, 54, 39–46.
- Mitaka, Y.; Mori, N.; Matsuura, K. A termite fungistatic compound, mellein, inhibits entomopathogenic fungi but not egg-mimicking termite ball fungi. Appl. Entomol. Zool. 2018, 54, 39–46.Citron, C.A.; Rabe, P.; Dickschat, J.S. The scent of bacteria: Headspace analysis for the discovery of natural products. J. Nat. Prod. 2012, 75, 1765–1776.
More
