Magnetomechanical therapy is one of the most promising areas in tumor microsurgery. The nanoscalpel can become a tool sufficient for cancer microsurgery. In order to achieve this, It should meet the following requirements: (1) to be nano- or micro-sized; (2) to have affinity and specificity for targets on tumor cells; (3) to have the ability for remote control. This nano- or microscalpel should include at least two components: (1) a physical nanostructure (particle, disk, plate) capable of converting a magnetic moment into a mechanical moment; and (2) a ligand molecule (antibody, aptamer, etc.) that allows the scalpel to accurately target tumor cells. The most suitable structures for nanoscalpel are anisotropic, magnetic micro- or nanodiscs with high saturation magnetization and no remanent magnetization. This type of material would facilitate remote control of the scalpel through a magnetic field. In addition, anisotropy enhances the transmigration of discs to the tumor. Today, four types of magnetic microdiscs are used for tumor destruction: synthetic antiferromagnetic P-SAF (perpendicular) and SAF (planar), vortex Py and three-layer non-magnetic-ferromagnetic-non-magnetic systems with flat quasi-dipole magnetic structures.
Magnetomechanical therapy is one of the most promising areas in tumor microsurgery. The nanoscalpel can become a tool sufficient for cancer microsurgery. In order to achieve this, It should meet the following requirements: (1) to be nano- or micro-sized; (2) to have affinity and specificity for targets on tumor cells; (3) to have the ability for remote control. This nano- or microscalpel should include at least two components: (1) a physical nanostructure (particle, disk, plate) capable of converting a magnetic moment into a mechanical moment; and (2) a ligand molecule (antibody, aptamer, etc.) that allows the scalpel to accurately target tumor cells. The most suitable structures for nanoscalpel are anisotropic, magnetic micro- or nanodiscs with high saturation magnetization and no remanent magnetization. This type of material would facilitate remote control of the scalpel through a magnetic field. In addition, anisotropy enhances the transmigration of discs to the tumor. Today, four types of magnetic microdiscs are used for tumor destruction: synthetic antiferromagnetic P-SAF (perpendicular) and SAF (planar), vortex Py and three-layer non-magnetic-ferromagnetic-non-magnetic systems with flat quasi-dipole magnetic structures.
- nanodiscs
- microdiscs
- magnetomechanical therapy
- magnetic field
- the nanoscalpel
1. Background
Malignant neoplasms remain one of the leading causes of mortality in the working-age population
and are an important public health problem
Despite the development of new methods for the diagnosis of oncological diseases and anticancer therapy, the proportion of deaths among all cancer patients exceeds 40%.
Currently, surgical resection and radiation therapy of tumors remain the leading physical methods for the removal or destruction of malignant neoplasms. The main disadvantage of these methods is their high invasiveness. Both surgery and radiation therapy damage the healthy tissue surrounding the tumor. This approach is dangerous, especially when treating brain tumors. Radical resection is not possible because individual tumor cells are invisible. The main task of radiation therapy is to maximize the radiation dose to the tumor while minimizing damage to the surrounding healthy tissue. Low doses of ionizing radiation can cause side effects, including inflammation, fibrosis, atrophy, vascular damage, hormonal deficiencies, and secondary malignancies
Therefore, there is a need for a new targeted approach capable of completely removing tumor tissue with minimal damage to the healthy environment. Nanoscalpel could become such an instrument. For that, it should have three main properties: (1) it must be miniature (nano- or micro-sized); (2) it must have affinity and specificity for tumor cells only; (3) it must be capable of being remotely controlled. The creation of such a tool is possible only with the help of nanotechnology, using nanomaterials with unique electronic, optical and magnetic properties. A nanoscale surgical instrument for tumor destruction should include at least two components (Fig. 1). The first component (the micro- or nanoscalpel itself) must be capable of damaging the tumor cell under the influence of external forces, causing the processes of its death. The second component should act as a recognizing element and interact only with its target, thereby bringing the nanoscalpel into contact with the tumor cell. In addition, the nanoscalpel should be able to be used with tumor cell imaging molecules, for example, a fluorescent dye or an antitumor drug.
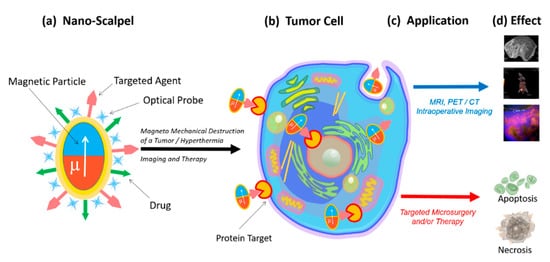
Fig. 1.
Schematic representation of a new nanoscalpel device (a): selective binding to tumor cells (b) for various applications (c), such as accurate diagnosis and targeted therapy (d).
2. Nanoscalpel
The magnetic field energy transformation is the most promising option for the destruction of tumor cells, since magnetic fields are safe for humans, and at the same time they can penetrate into the body and control magnetic structures [7]. Depending on the characteristics of the magnetic field, its energy can be converted into either heat energy, causing the particles heating and hyperthermia of the tumor tissue [4], or into mechanical energy, causing the oscillation or rotation of the magnetic particles, leading to the mechanically induced cell programmed death or necrotic damage [8][9].
In order to perform direct mechanical damage, the torque of the magnetization must be translated into a force applied to the cell, which is capable of damaging the cell membrane [10][11] or organelles (in case of penetration of magnetic particles into the cell) [7][12][13][14][15].
The magnetic field creates magnetization, which due to magnetic anisotropy, becomes a torque for a physical particle. This torque can be used in a variety of ways. The most attractive way to destroy tumor cells for microsurgery is magnetomechanical destruction due to a magnetic field’s action. Magnetomechanical transduction can act on death receptors [16], ion channels [17], or can directly damage the cell [10][13][15]. This effect has been shown both
Superparamagnetic iron oxide nanoparticles (SPION), which exhibit magnetic properties only when a magnetic field is applied, are the most popular ones [15]. Without a magnetic field, the magnetic moment of the nanoparticles equals zero. Such nanoparticles are often used for magnetic resonance imaging, hyperthermia induction, and mechanical destruction of cells [4][15][18]. However, the effectiveness of these nanoparticles in destroying tumor cells has reached its limit [19]. Their size restricts the magnitude of the SPION magnetic response required for biomedical applications, since nanoparticles aggregate above the superparamagnetic threshold [19].
Usually, magnetic nanoparticles have a magnetic core, protective shell, and a biologically functional coating. Ferromagnets, ferrimagnets, and superparamagnets are used as magnetic materials. Various protective materials are used for MNP coatings for biomedical research, since uncoated magnetic particles are unstable under physiological conditions [20][21]. In addition, they contribute to the formation of free radicals [22], can agglomerate [23], and can be opsonized and captured by macrophages [24].
For targeted delivery, MNPs are functionalized with ligands capable of specifically binding to target cells. Peptides, antibodies, aptamers, and small molecules with affinity and selectivity are used as carriers. Ligands increase the circulation time of magnetic particles in the blood and improve their biocompatibility [25][26][27].
The use of a nanoparticle-based magnetomechanical approach using a low-frequency, non-heating magnetic field demonstrated the ability of magnetic nanoparticles to convert the energy of a magnetic field into deformation and to create a change in the conformation of macromolecules attached to them [28]. Thus, MNPs turned out to be capable of manipulating cellular functions with mechanotransduction using remote control of the magnetic field [29][30]. In magnetic fields, MNPs can stimulate or suppress cellular functions such as apoptosis, differentiation, migration, proliferation, and secretion [31][32]. An alternating magnetic field can cause oscillations of magnetic nanoparticles and can form the basis of magnetomechanical remote destruction of tumor cells [30]. Successful destruction of tumor cells using MNPs functionalized with antibodies under the action of an alternating magnetic field has been demonstrated
in vitro
in vivo
in vivo tumor destruction with aptamer-functionalized MNPs [34]. However, superparamagnetic nanoparticles are primarily used to induce hyperthermia in tumor tissues [30][35][36][37][38]. This approach is based on the high sensitivity of tumor tissues to slight increases in temperature. Heating the tumor up to only +42 °C causes irreversible disruption of the protein conformation due to the tumor tissue’s higher acidity. In contrast, the proteins of normal tissues are insensitive to this temperature [39]. From a physical point of view, MNPs convert magnetic energy into heat during their magnetization reversal in high-frequency magnetic fields. Magnetic nanoparticles suitable for hyperthermia have a high specific absorption rate (SAR), allowing them to heat up quickly in alternating magnetic fields. However, the efficiency of converting magnetic field energy into thermal energy is low, and the MNPs doses or magnetic anisotropy have to be increased [40][41].
3. Properties of Magnetic Nano- and Microdiscs
To date, four types of magnetic discs are used for tumor cell destruction: synthetic antiferromagnetic SAF (in-plane) and P-SAF (perpendicular) [5][42][43][44], vortex Py [10], and three-layer non-magnetic–ferromagnetic–non-magnetic systems [45]. The pronounced magnetostrictive properties and small crystallographic anisotropy make nickel a preferable ferromagnetic layer in the disc’s center [17] (

Figure 2.
The first type of synthetic antiferromagnetic disc consists of two ferromagnetic layers separated by a non-magnetic layer. The magnetic layers become interconnected and behave like single magnetic moments with opposite directions of magnetization, compensating each other. When an external magnetic field is applied, the magnetic moments are oriented in one direction, reaching saturation. Synthetic antiferromagnetic nanoparticles have unique magnetic properties [43]. The thickness and material of the non-magnetic spacer controls the exchange coupling between the ferromagnetic layers, causing these layers to magnetize in opposite directions. As a result, the structure with zero net magnetization is easily switched in an external field. By varying the thickness of the layers that make up the disk and obtaining the magnetic susceptibility, it is possible to control the dispersion of particles in the solution [46]. P-SAF disks are similar to SAF disks, but their magnetization is perpendicular to the plane of the disk [47].
Vortex disks are characterized by the fact that magnetic moments are twisted in the plane of the disk and are located in closed circles to minimize magnetostatic energy. Only in the core do the magnetic moments leave the plane, where they are directed perpendicularly. This rotation pattern also has zero residual force. When an external magnetic field is applied, the vortex core shifts in the plane of the disk until it reaches the edge where it annihilates and the disk becomes magnetically saturated [10].
To cause vibration or oscillation of the particles, the applied magnetic field should rotate or change with sufficient amplitude, preferably close to the saturation field of the particles. The key parameter is the magnetic anisotropy of the particle, which relates the moment of magnetization to the mechanical moment on the particle. The diameter and thickness of the magnetic disks can be optimized to obtain the desired configuration of the magnetic vortex [48]. A planar magnetic structure would provide low residual magnetization, sufficient magnetic susceptibility for effective magnetic excitation, and insufficient agglomeration [44][49][50][44,49,50].
Nano- and microdiscs are made by methods that combine lithography (usually photolithography or electron beam) and physical deposition of materials (sputtering or thermal evaporation). The ease of controlling the manufacturing conditions of the discs makes the process automatic and repeatable. The shape and simplicity of manufacture, including one-stage metal deposition on a patterned photoresist layer, make it possible to reduce the size of the discs to 100 nm while maintaining the vortex state [51][52][51,52]. The disc sizes can be further reduced using the latest advances in nanoimprinting and deep UV lithography [53]. The methods used for the manufacture of synthetic antiferromagnetic and vortex disks and disks with a flat quasi-dipole magnetic structure allow strict control of their shape, size and composition, making the manufacturing process reproducible.
4. Biological effects of discs on tumor cells in an alternating or rotating magnetic field
A fundamental property of all living organisms is mechanosensitivity, which allows cells to perceive physical signals and mechanical forces generated in their microenvironment. This technique controls the parameters of the internal and external environment of the body. Mechanoreceptors control the functional state of cells, tissue growth, stem cell differentiation, apoptosis and necrosis [54][55][56][54,55,56]. Therefore, the mechanosensitivity of cells, including tumor cells, provides the possibility of external control of their functional state.
Vortex and artificial antiferromagnetic (SAF, P-SAF) magnetic nano- and microdisks and disks with a flat quasi-dipole magnetic structure are promising tools for remote control of the functional state of cells. In a low frequency rotating or alternating magnetic field, discs can convert magnetic energy into mechanical energy. Currently, micro- and nanodiscs with zero magnetization have begun to be used in experiments to modulate cell function in the process of bone tissue regeneration [32] and destruction of tumor cells [10].
in vitro
in vivo
Table 1.
| Size | Disc Type | Composition | Magnetic Field Characteristics | Biological Effect In Vitro | Reference |
|---|
| 2 μm | P-SAF | CoFeB connected by Pt/Ru/Pt spacers | Rotating magnetic field, 10 kOe Duration 1 min Torque 18 nN |
Destruction of 62% of U87 cells | [53] | |||||
| 2 μm | Py | Ni | 80 | Fe | 20 | Rotating magnetic field, 10 kOe Duration 1 min Torque 75 nN |
Destruction of 12% U87 cells | [53] | ||
| 150/200/350 nm | Py | Ni | 80 | Fe | 20 | Alternating magnetic field, 20 HzDuration 2 h | Destruction of 83.4/83.2/82.5% of HeLa cells | [49] | ||
| 2 μm | P-SAF | (Ta/Pt/CoFeB/Pt/Ru/ PT/CoFeB) | 10 | Rotating magnetic field, 1 T Duration 20 min |
Destruction of 70% of U87 cells | [7] | ||||
| 1.3 μm | Py | Ni | 80 | Fe | 20 | Rotating magnetic field, ∼20–30 mT, 20 Hz Duration 1 h |
Destruction of 70% of renal cancer cells | [17] | ||
| 1 μm thickness 60 nm |
Py | Ni | 80 | Fe | 20 | Rotating magnetic field, 9 mT 20 Hz Duration 10 min |
Destruction of 90% of human glioma tumor cell line No. 10 cells ( | N | 10) | [10] |
| 2 μm | Py | Ni | 80 | Fe | 20 | Rotating magnetic field 1 T, 20 Hz, Duration 30 min |
Destruction of 60% of U87 cells. In vivo survival is 3 times higher, and the tumor is 3 times smaller |
[7] | ||
| 0.14 μm |
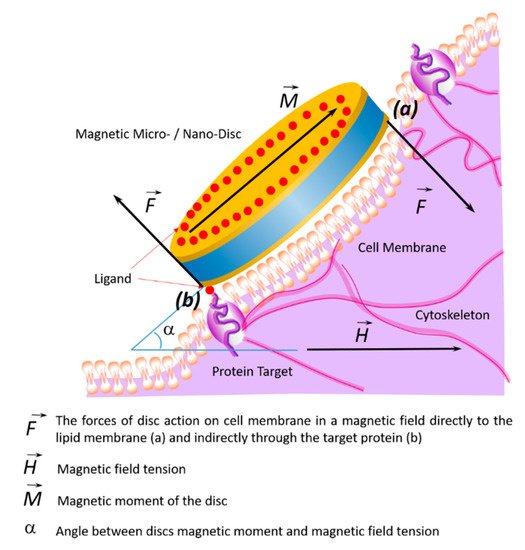
Figure 4.
a
b
8. Biological Effect of Magnetic Discs In Vivo
in vitro
in vivo
et al.
9. Transfer of Magnetic Discs along with the Bloodstream
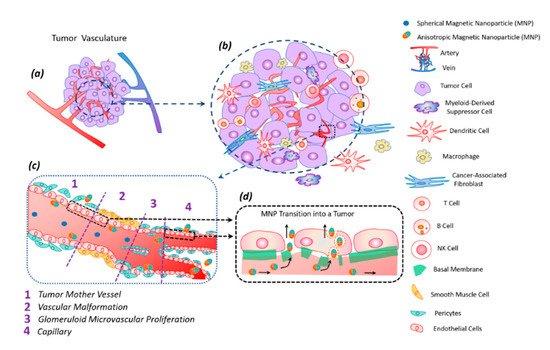
Figure 5.
a
b
c
c
d
The anisotropic shape of the magnetic particle facilitates its better penetration into the tumor [62]. The vibration of anisotropic particles due to hydrodynamic or magnetic forces causes enhanced particle interactions with the vessel wall and transmigration into the tumor [43][62]. The vascular density of a tumor is usually the highest at the tumor–host interface. Central parts of tumors tend to be less vascularized, which often have zones of necrosis due to insufficient blood supply. Plasma proteins increase the viscosity of the blood, slowing down the blood flow. For this reason, nanoparticles within the tumor vessels tend to move slowly or stagnate. Therefore, nanoparticles have enough time to diffuse from the vessel into the tumor’s extracellular matrix. Size is an important parameter for magnetic particles—they should be small enough not to clog blood microcapillaries and pass through the pores of blood vessels in order to diffuse into tissues.
10. Modification and Functionalization of Discs
The stability of magnetic discs could also be increased through their functionalization with specific ligands, promoting targeted action on a tumor cell. Additionally, to reduce side effects, the magnetic particles must only enter the tumor cells. Additional functionalization with the ligands specific only to tumor cells, such as vascular endothelial growth factor receptor (VEGFR), transferrin receptors, or integrins, is required for the targeted action [63][64]. Additionally, the optimization of the target biodistribution of magnetic discs in vivo will be determined by the local blood flow, pH, organization of the vascular network, and extracellular matrix.
Table 2.
| Disc Type | Recognizing Agent | Disc Binding to Recognizing Agent | Cell Type; Destruction Rate | Reference |
|---|
| The 60-nm-thick, ~1-μm-diameter 20:80% iron–nickel (permalloy) discs, coated with a 5-nm-thick layer of gold on each side | Antibodies anti-IL13 | α | 2R | S–Au bond | Human glioma | N | 10 cell line; 90% (in vitro) | [10] |
| The 60-nm-thick, ~1-μm-diameter 20:80% iron–nickel (permalloy) discs | Antibody antihCA9 | S–Au bond | Renal SCRC-59 renal cancer line; 90% (in vitro) | [17] | ||||
| Discs with a flat quasi-dipole magnetic structure Au/Ni/Au |
Aptamer | S–Au bond | Ehrlich ascites adenocarcinoma cell line; 80% (in vitro, in vivo) | [18] | ||||
| Discs with a flat quasi-dipole magnetic structure Au/Ni/Au |
Aptamer | S–Au bond | Ehrlich ascites adenocarcinoma cell line; 90% (in vitro, in vivo) |
[45] | ||||
| Py | ||||||||
| Ni | ||||||||
| 80 | Fe | 20 | Rotating magnetic field 10 mT, 20 Hz Duration 30 min |
Destruction of 60% of cells | [57] | |||
| 2 μm | Py | Ni | 80 | Fe | 20 | Rotating magnetic field 10 mT, 20 Hz Duration 30 min |
Destruction of 12% of cells | [57] |
| 1 μm | Discs with a flat quasi-dipole magnetic structure | Au/Ni/Au | Rotating magnetic field, 50 Hz 5 mT Duration 20 min In vitro In vivo |
Destruction of 80% of Ehrlich ascites adenocarcinoma cell | [18] | |||
| 1 μm | Discs with a flat quasi-dipole magnetic structure | Au/Ni/Au | Alternating magnetic field, 50 Hz, 5 mT Duration 20 min |
Destruction of 90% of Ehrlich ascites adenocarcinoma cell | [45] |
et al.
in vitro
in vitro
in vivo [7][14][17]. Simultaneously, vortex nanodiscs (140 nm) were more effective in the destruction of tumor cells than vortex microdiscs (1 μm) [57].
et al.
et al.
5. The Mechanism of Tumor Cell Death Exposed to Discs under the Influence of a Magnetic Field
The death of tumor cells using micro- and nanodiscs can occur as a result of necrosis or apoptosis. Necrosis occurs due to mechanical destruction of the cell membrane (magnetoporation) or destruction of the entire cell (magnetolysis). Necrosis is an unfavorable method of tumor destruction, since it causes inflammation due to lysosomal enzyme release. Therefore, the most favorable tumor cell elimination pathway is apoptosis, which is suppressed in cancer cells due to oncogenic mutations. The inflammatory process does not accompany apoptosis; therefore, all researchers are looking for magnetic field characteristics stimulating apoptosis in tumor cells [10][17]. In the magnetic field, discs induce cell death in two ways: (1) by internalizing into the cell (
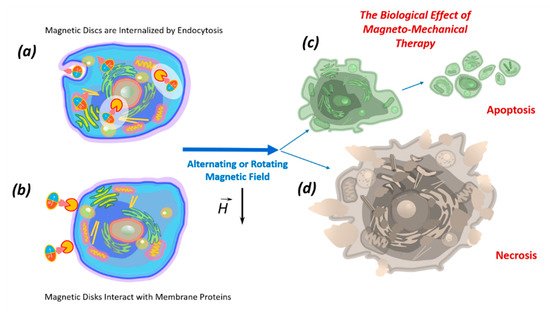
Figure 3.
a
b
c
d
6. Internalization of Magnetic Discs
80
20
7. Impact of Discs on the Cell Membrane
The effects of the discs on the cell membrane of tumor cells and their internalization into the cell occur due to their binding to membrane proteins. This effect of the magnetic discs on tumor cells induces programmed cell death (apoptosis) [10][58][59]. The binding of discs to the cell membrane occurs due to their functionalization via recognition molecules — either antibodies or aptamers. Antibodies to carbonic anhydrase CA9, which is overexpressed on the cell surfaces of solid tumors, were used to functionalize the discs. Magnetic discs with anti-CA9 induced apoptosis in 70% of tumor cells under the influence of a magnetic field [17]. Using magnetic discs functionalized with antibodies to IL13α2R, other authors changed the homeostasis of calcium cations in tumor cells, stimulating apoptosis [10]. In this case, the stimulation of mechanosensitive cell receptors using vibrating discs caused the cell membrane to stretch, which led to an increase in the intracellular level of calcium cations [59]. Presumably, the cytoskeleton, which responds to external or internal physical stimuli and is responsible for cellular mechanical signal transmission, cell shape regulation, and migration, plays a key role in controlling the cell’s functional state in external magnetic fields. A schematic diagram of the target cell’s functional state is shown in
11. Toxicity of Magnetic Discs
The mechanism of the toxic action of MNPs is not fully understood, however, it is assumed that it is primarily due to oxidative stress [68] caused by the formation of reactive oxygen species (ROS) in the Fenton reaction Fe2+ + H2o2 = Fe3+ + OH• + OH− during the interaction of magnetite with cage [69][70][69,70]. Iron ions with ROS are formed during the destruction of magnetite in lysosomes. In addition, ROS can be formed from the surface of MNP by leaching metal ions or by the release of oxidants by enzymatic degradation of MNP. The resulting hydroxyl radicals react with DNA, proteins, polysaccharides, and lipids. Accumulation of ROS destroys cellular proteins, enzymes, lipids, and nucleic acids, promotes disruption of cellular metabolism, and leads to apoptosis and necrosis [71]. Low concentrations of superparamagnetic iron oxide nanoparticles protect cells from damage and death caused by oxidative stress [72][73][72,73]. In particular, nanoparticles 10 nm in size at concentrations of 10–30 μg/ml did not induce cytotoxicity in HeLa cells and did not affect the behavior of rats, nor were they toxic to the liver, kidneys, lungs, or spleen [74].
3
4
MNPs with a diameter of 18 nm stabilized with citrate ions at concentrations from 50 μg/ml to 2 mg/ml did not reduce the survival of human liver carcinoma cells
5-6 nm in size and stabilized with citrate ions, PEG and glucose at concentrations up to 1 mg/ml did not affect the survival of HeLa cells [76], while 35 nm Fe3O4@Au MNPs stabilized by citrate ions at a concentration of 1 mg/ml concentration did not affect the survival of murine fibroblasts [77]. It was found that MNPs enter cells without disrupting the membrane or cytoskeleton. In general, the toxic effect of nanosized particles is due to their high reactivity, effective diffusion through biological membranes, and the ability to overcome tissue barriers [69].
Magnetic fields are attractive for therapy, as they can penetrate the entire depth into the internal organs and tissues of the body. The biosafety of magnetic fields for magnetodynamic therapy is extremely important. Most of the fields used for theranostics are practically safe [78].
Numerous studies have shown that magnetic nano and microdiscs internalized into a cell, regardless of their number in the absence of a magnetic field, do not exhibit cytotoxicity and do not affect cell viability [5][20][5,20]. However, data on the effect of magnetic disks on cell proliferation are ambiguous.
The selective action of nanodiscs and MNPs on target cells due to aptamers significantly increases their biocompatibility and reduces their toxicity [27].
Thus, it can be concluded that microsurgery of malignant tumors using a nanoscalpel based on functionalized tumor-recognizing ligands is low-toxic.
