Steroids are a pivotal class of hormones with a key role in growth modulation and signal transduction in multicellular organisms. Synthetic steroids are widely used to cure large array of viral, fungal, bacterial, and cancerous infections. Brassinosteroids (BRs) are a natural collection of phytosterols, which have structural similarity with animal steroids. BRs are dispersed universally throughout the plant kingdom. These plant steroids are well known to modulate a plethora of physiological responses in plants leading to improvement in quality as well as yield of food crops. Moreover, they have been found to play imperative role in stress-fortification against various stresses in plants. Over a decade, BRs have conquered worldwide interest due to their diverse biological activities in animal systems.
1. Introduction
In living systems, biochemical interaction among cells is achieved by chemical messengers that pass between them. These chemicals serve to coordinate the metabolic activities of various tissues and also allow individuals to adapt to changing environments and prepare them for reproduction. One of the major classes of chemical messengers is known as hormones. In animals, hormones are synthesized in specialized ductless glands and are carried by the circulatory system to other parts of the body. On the other hand, plants have the capability to synthesize hormones, which is rather widely scattered in cells. Although, the site of synthesis varies as the plant develops and the hormones may only be translocated relatively short distances
[1][2][1,2]. Steroids, a crucial category of hormones, play an essential function in plant signaling responses and modulate a plethora of physiological and growth attributes. Several sex hormones, steroidal in nature, have also been reported in plants such as corticosteroids, estrone, progesterone, testosterone, and large number of analogues of these compounds
[3][4][3,4]. Exogenous supplementation with these steroidal sex hormones results in significant modification of morphological and physiological responses in plants and animals
[5][6][7][5,6,7].
Acetate is primary precursor for synthesis of cholesterol. It is postulated that three acetate molecules combine to form a single five carbon unit called isoprene. Cholesterol is an acyclization product of squalene, a 30-carbon polymer made up of six isoprene units. Squalene could readily be converted into cholesterol involving the synthesis of lanosterol through many steps. Cholesterol after a series of side chain cleavages and oxidations is converted to Delta-5-pregenolone which is further converted to progesterone by a dehydrogenase or to 17α-hydroxy-pregenolone by a specific 17-hydroxylase. Biosynthesis of steroids in plants is similar to that in animals. Different species are able to transform cholesterol to several steroids, for example, cholesterol is transformed to pregenolone by
Digitalis species
[8][9][10][8,9,10] and by
Haplopappusheterophyllus [11]. The
Digitalis lanata plants also transform sitosterol to progesterone
[12]. Progesterone is then converted to corticosterioid by
D. lanata [13]. The
Mallototuspaniculatus plants alter cortisol to cortisone
[14]. Similarly, observation revealed transformation of cholesterol and sitosterol, applied on plants, to a wide array of steroid hormones present in animals. The observations revealed that certain plants are able to synthesize Delta-4-3-oxo-steroids
[15].
A novel group of steroidal hormones was discovered in 1970, which had structural similarity with animal steroidal hormones. BRs are polyhydroxy steroidal hormones, first isolated by Grove et al.
[16] from pollens of
Brassica napus and were referred as brassinolide (BL). The second BR isolated was castasterone (CS) by Yakota et al.
[17]. Since then, 70 different BRs (five conjugated and 65 un-conjugated) have been identified in various plant species
[18][19][18,19]. The endogenous content of BRs varies from tissue to tissue as well as in micro-molar and nano-molar concentrations
[20][21][22][20,21,22]. The immature seeds of plants and their pollens have been reported to have the highest concentration of BRs in them and are also dependent upon the age of plants
[23][24][23,24]. Like their counterparts or similar analogue, BRs have a wide array of responses in various developmental and physiological functions of plants such as germination, regulation of gene expression, cellular and vegetative growth, differentiation of vasculature, root growth, apoptosis, homeostasis, and reproductive development
[25][26][27][28][25,26,27,28]. BRs not only have an imperative role in plant development at the organ level but also at the cellular level. It has been reported to trigger fission and elongation of cell, activate protein and nucleic acid synthesis, elevate photosynthetic efficiency, structural changes in the cell wall, and modulation of permeability of the cell membrane
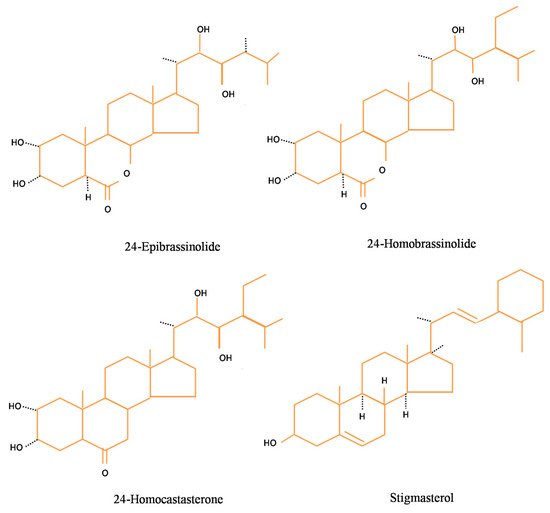
[29]. depicts the structures of certain basic brassinosteroids viz. 24-EpiBL, 28-HomoBL, 28-Homocastasteone, and stigmasterol.
Figure 1. Structure of basic brassinosteroids.
Moreover, BRs also play a dynamic role in plant acclimatization in response to varied stresses
[30][31][32][30,31,32]. External supplementation of BRs triggers innate potential of plant to survive against stresses, specifically receding biotic stresses caused by variable pathogens including fungi, bacteria, and viruses
[33][34][35][33,34,35]. Application of BRs has also been found to control cell proliferation and promote cell death of cancer cells in various mammals, including humans
[36][37][38][36,37,38]. Beside anticancerous effects, they possess antiviral, antifungal, antibacterial, and ecdysteroidal properties. They have also been observed to heal cutaneous wounds by controlling inflammatory and re-epithelialization processes
[39].
2. Therapeutic Role of BRs
Recent development in studies related to bioactivities of BRs in several animal models indicates their significant potential in therapeutic applications. They are suggested as anticancerous, antiproliferative, antiangiogenesis, antiviral, antigenotoxic, antifungal, and antibacterial, and display anti-chloesterolemic activities, synthetic medication
[36][40][41][42][36,52,53,54] which are briefed below:
2.1. Anticancerous/Antiproliferative Activities
Sterols with oxygenated side chains have been procured from several plants and marine organisms which are toxic to mammalian cancerous cells
[43][55]. BRs are known to have therapeutic characteristics against cancer development and show potential for developing new anticancer drugs
[36][44][45][46][36,56,57,58]. Several studies further reported that a BRs analogue with similar oxygenated side chain had similar activities as that of sterol
[47][48][59,60]. Cancer cells have distinctiveness of indefinite proliferation and not undergoing apoptosis normally. BRs can stimulate obligatory induction of necessary responses required for inhibition of growth and stimulation of apoptosis by interfering with the cell cycle
[36]. Various BRs have been studied for anticancer bioactivities in various cell lines i.e., CEM (T-lymphoblastic leukemia), A-549 (lung carcinoma), MCF-7 (breast carcinoma), RPMI 8226 (multiple myeloma), HeLa (cervical carcinoma), MDA-MB-468 (breast carcinoma), and LNCaP (prostate cancer) etc. All these cell lines were observed for viability test after six serial 4-fold dilutions for 72 h. IC
50 value of BRs as observed from Calcein AM assay gives an idea of their potential to be used as anticancer drugs
[36].
As reported by Malikova et al.
[36], 28-homoCS, CS and its homologue
22S,23S-28-homocastasterone showed maximum cytotoxicity for cancer cell lines and BRs showed a different kind of response in different cells as no viability loss was observed in the fibroblast cells. The effect of BRs (28-homocCS and 24-EpiBL) was studied on viability of these human cancer cell lines (CEM and RPMI 8226). It was reported that both (28-homoCS and 24-EpiBL) reduced the viability of CEM and RPMI 8226 in a dose dependent manner even at micro molar concentration and 28-homocastasterone was more active and potent for CEM cells
[36]. These two BRs were also analyzed for the anticancer effects in the breast cancer cell lines MDA-MB-468 (estrogen receptor-α-negative) and MCF-7 (estrogen receptor-α-positive), and prostate cancer cell lines DU-145 (androgen-insensitive), and LNCaP (androgen-sensitive). The androgen and estrogen (sensitive and insensitive) cell lines behave diversely when exposed to the BRs. Most of prostate cancer cells have both sensitive and insensitive types of cells and are required for the elimination of cancer
[49][50][61,62]. It has been reported that hormone responsive cell lines could be more efficiently treated with BRs. Hence, the research has been directed towards the probable inflection of steroid receptor-mediated response of BRs and development in many test models applied for advancement of new BR derived anticancerous drugs
[33]. 28-homoCS and 24-EpiBL influence proliferation, survival, and cell cycle of breast cancer cell lines (DU-145 and LNCaP). Cell growth is inhibited by both BRs in a dose dependent manner in cancerous cell lines. Analysis by flow cytometry showed that treatment of BR inhibited MDA-MB-468, MCF-7, and LNCaP cells in G
1 phase and stimulates cell death in LNCaP, MDA-MB-468, and minutely in DU-145 cells resulting in reduction of cell percentage in S-phase. In response to application of antiestrogen, MCF-7 cancerous cell lines suppressed cell proliferation resulting in reduced quantity of cells synthesizing DNA (S phase) and shifts to G
o/G
1 phase
[51][63]. It was reported by Oklestkova et al.
[37] that BRs suppress the growth of varied cancerous cell lines at µM levels without altering normal cell growth. BRs are applied to cure disorders, and a few of them involve proliferation of cells and include Huntington disease, Alzheimer disease, sexual differentiation disorders, cancer, and steroid induced osteoporosis, hyperadrenocorticism linked with excess of sex steroids, steroid induced cataract, glucocorticoid insensitive asthma, and P450 oxidoreductase deficiency. BRs influences differentiation, proliferation, survival, and expression of certain cell cycle associated proteins in cancerous cell lines
[52][64].
The mechanism of antiproliferative activity of 28-homoCS and 24-EpiBL was studied by Steigerova et al.
[53][54][51,65] in prostate cancer cell lines, DU-145 and LNCaP (hormone insensitive and sensitive). Various techniques like flow cytometry, TUNEL DNA ladder Test, Western blotting, and immunofluorescence were used to study the effect of these BRs on prostate cancer cells. Repression of cell growth and blockage at G
1 phase along with decline in CDK4/6, cyclin D1, and pRb expression was recorded in LNCaP cells. However, in DU-145 cells a high proportion of cells were observed in the G
2/M phase along with reduction of expression cyclins A and B
1. Apoptosis initiated by BRs was observed with an increase in the subG
1 fractions in DU-145 and LNCaP cell lines in TUNEL staining and identification of DNA (low molecular weight) recognized by bands visible in Western blotting.
BRs are recommended to cure the harmful effects of hyper proliferation in mammalian cells in vivo as well as in vitro, especially to cure hyper proliferative disorders. 28-homoCS also reduces the expression of p53, p27, p21, Bcl-2 family anti-apoptotic proteins, cyclins, and ER-alpha (estrogen receptor α)
[33]. A 24-EpiBL treatment (10
−6 to 10
−9 mol L
−1) modified growth properties of mouse hybridoma cultured under nutritional stress due to growth in either standard medium (serum free) or 30% diluted medium. Enhancement of mitochondrial membrane potential, number of cells in G
0/G
1 phase and S phase, and reduction of intracellular antibody have been reported by 24-EpiBL treatment.
The anticancer activity of EBR against prostate cancer cells by inducing apoptosis in androgen sensitive cells (LNCaP) was investigated using SILAC (stable-isotope labeling by amino acids in cell culture). The cancer cells, prior to 24-EpiBL treatment, were quantified for proteins and it showed that expression of calreticulin (CALR) an unfolded protein response (UPR) chaperone protein was under expressed and initiated the CHOP (an antibody against ER stress) translocation from the cytoplasm to nucleus. This CHOP translocation activates the caspase-9 and caspase-3 enzymes. Co-application of 24-EpiBL with rapamycin (a well-known translational inhibitor) inhibits the cell death and PARP cleavage in LNCaP cells suggesting that 24-EpiBL could induce ER stress in these cells
[55][66]. However, in another study 24-EpiBL treatment has been found to enhance the cell death in prostate cancerous cell lines (LNCap and DU 145) by increasing the expression of PAO (polyamine oxidase) and SSAT (spermine N1-acetyltransferase) enzymes
[55][66]. Brassinolide has been observed to promote apoptosis of human prostate cancer cell line, PC-3, by enhancing caspase-3 activity and downregulating the expression of Bcl-2 (an anti-apoptotic protein)
[56][67]. Application of 24-EpiBL in colon carcinoma cells (HT-29 and HCT 116) have been found to upregulate Foxo3a (Forkhead/Winged Helix Box Class O) and protein tyrokinase Src p38, after the activation of PI3K/AKT, hence leading to mitochondria-regulated cell death in colon cancer cells
[57][68]. Antiangiogenic property of the BRs and its derivative (cholestanon) was checked in human micro vascular endothelial cells (HMEC-1) and human umbilical vein endothelial cells (HUVEC). It was observed that 24-EpiBL and 28-HomoCS inhibited proliferation of HMEC-1 cells in a concentration dependent manner and also inhibited migration of HUVEC cells and cholestogen strongly decreased the cell adhesion, affecting antiproliferative activity of the cells. The weakest opponent of estrogen-receptor-α (ERα) was found to be 24-EpiBL. The results showed that BRs and its analog BR4848 can suppress in vitro angiogenesis of primary endothelial cells and inhibit angiogenesis and prevent the development of metastasis
[58][59][50,69].
2.2. Antiviral Activities
The primary facet of the defensive role of BRs is associated with their capability to provide resistance against viruses
[60][61][70,71]. The first line of inner resistance to stress is swiftly induced by host recognition by presence of conserved structural motifs which is entirely articulated by pathogens. These motifs are recognized as PAMPs and are discharged by the host throughout pathogen attack or DAMPs i.e., damage-associated molecular patterns discharged from wound
[62][72]. The detection of various DAMPs/PAMPs by particular sensors present on the cell surface, known as PRRs, stimulates a refined defense signaling mechanism which hampers a wide range of potential pathogens such as viruses, bacteria, oomycetes, and fungi. PRRs are selected by receptor-like proteins and receptor-like kinases situated on the surface of the cell. Receptor-like kinases/proteins require a co-receptor in order to generate an activated complex to start signaling. BRASSINOSTEROID INSENSITIVE (BRI1)-related kinase BAK1 is the best classified co-receptor of PTI which produces active signaling complexes with receptor-like kinases as well as receptor-like proteins after PAMP recognition through PRRs
[63][73]. Interaction of PRRs and LRR-RLK BRI1 with BAKI is directly proportional to availability of ligands. BAK1 is imperative for plant immunity under RNA virus infestation.
bak1-4 and
bak1-5 mutants were reported to have higher levels of TCV, ORMV, and TMV accumulation when compared to wild plants
[64][74]. BR treatment significantly decreased virus infection and enhanced the crop yield by 56%. Tobacco plants treated with brassinolide (BL) induced resistance against TMV
[65][75]. BAK1 or BKK1 are essential components in controlling Turnip crinkle virus infection in
Arabidopsis thaliana [66][76]. BRs supplementation increased antioxidative enzyme activity, regulated the defense associated gene expression, and reduced photosystem deterioration in
A. thaliana under cucumber mosaic virus infection
[67][77].
In
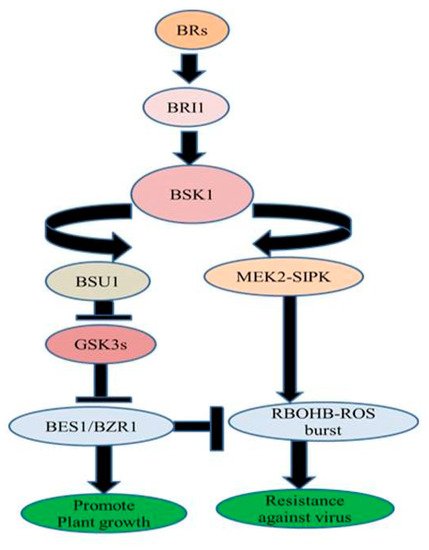
Nicotiana benthamiana, exogenous treatment of BRs increased resistance against TMV through MEK2 (MAPKK)-SIPK (salicylic acid-induced protein kinase) and RBOHB (respiratory burst oxidase homolog protein B)-dependent ROS burst. BES1/BZR1 suppressed RBOHB-dependent ROS generation and function as an essential mediator in BR signaling between growth and immunity. When the activated form of BZR1/BES1 is present in low concentration, RBOHB-dependent ROS burst generation is regulated by MEK2-SIPK signaling network which might have offered resistance to TMV. On the other hand, when the active form of BZR1/BES1 is present in elevated concentrations subsequent enhanced BR signaling might have inhibited RBOHB-dependent ROS formation by BZR1/BES1 and stimulated plant development ()
[68][78].
Figure 3. BR-regulated virus resistance.
Antiviral properties of BRs in response DNA and RNA viruses are tabulated in .
Table 1. Antiviral properties of BRs in response DNA and RNA viruses.
| Viruses |
(% Inhibition) |
| 28-homocastaterone |
Brassinolide |
| Poliovirus type 1 (RNA virus) |
85 |
96 |
| Vesicular stomatitis Virus Indiana strain (RNA virus) |
23 |
100 |
| Herpes simplex virus-1 F strain (tk | + | ) (DNA virus) |
50 |
96 |
| Herpes simplex virus-1 B2006 strain (tk | − | ) (DNA virus) |
35 |
100 |
| Herpes simplex virus-1 G strain (tk | + | ) (DNA virus) |
48 |
98 |
| Junin virus IV | 4454 | strain (RNA virus) |
79 |
74 |
| Tacaribe virus TRLV | 11573 | strain (RNA virus) |
99 |
55 |
| Pichinde virus AN | 3739 | strain (RNA virus) |
98 |
67 |
| Measles virus Brasil/001/91 (RNA virus) |
50 |
100 |