1. Introduction
Nitrogen-containing heteroaromatic rings are valuable motifs in bioactive molecules and recurrent scaffolds present in drugs [1][2]. The application of nitrogen ring systems in drug development is related to their diverse properties, including relatively small conformational freedom, while retaining some polarity, compared to aromatic hydrocarbons. Additionally, commercial availability, synthetic tractability, chemical diversity and the tendency for functionalization should also be highlighted [3]. However, the wide chemical space of nitrogen heterocycles is not yet fully explored in the attempt to find new drug candidates.
Nitrogen-containing heteroaromatic rings are valuable motifs in bioactive molecules and recurrent scaffolds present in drugs [1,2]. The application of nitrogen ring systems in drug development is related to their diverse properties, including relatively small conformational freedom, while retaining some polarity, compared to aromatic hydrocarbons. Additionally, commercial availability, synthetic tractability, chemical diversity and the tendency for functionalization should also be highlighted [3]. However, the wide chemical space of nitrogen heterocycles is not yet fully explored in the attempt to find new drug candidates.
Dihydropyrrolo[1,2-
a
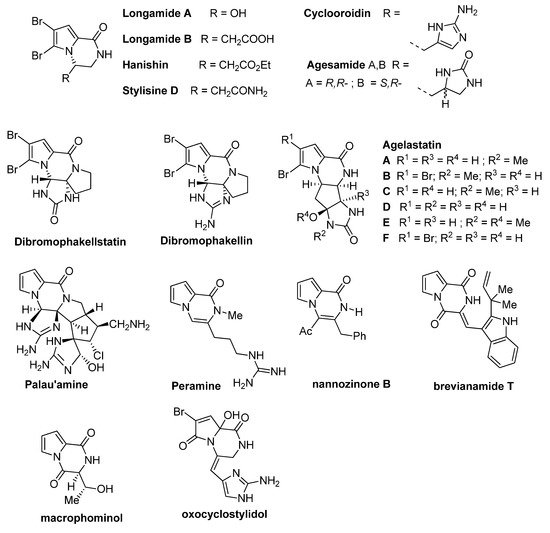
]pyrazinone rings are found in the structure of a number of bioactive compounds, including synthetic and natural products isolated from various sources like fungi, plants or sponges. These natural products (some structures are shown in
) often contain one or two bromine substituents on the pyrrole ring. The simplest congeners are
longamide A
[4] and its nonbrominated analog mukanadin C
longamide B
hanishin
stylisine D
cyclooroidin
agesamide
[10]. More complicated tetracyclic analogs include dibromophakellstatin
,
dibromophakellin
agelastatins A-F [12][13]. One of the most complicated pyrrolopyrazinone natural products is
[12,13]. One of the most complicated pyrrolopyrazinone natural products is palau’amine
[14], and its structure has been seen as a challenge for total synthesis. Some related natural products are the higher oxidation state analogs peramine
nannozinone B
[16], containing the pseudoaromatic pyrazinone ring, the pyrrolodiketopiperazines brevianamide T
macrophominol
[18], and the oxopyrrole derivative oxocyclostylidol
).
Figure 1.
Pyrrolopyrazinone natural products.
Several bioactivities have been found for these pyrrolopyrazinone natural products. Hanishin shows cytotoxicity against non-small cell lung carcinoma [7], and agelastatin A and D display significant activity against different cell lines [20]. Longamide B was found to have antiprotozoal [21] and antibacterial [6] properties, with good potency against African trypanosome. Palau’amine and the similar dibromophakellin and dibromophakellstatin inhibit the human 20S proteasome [22]. Peramine is an insect feeding deterrent [23].
2. Fusion of a Pyrazinone to a Pyrrole Derivative
2.1. Starting from 2-Monosubstituted Pyrroles
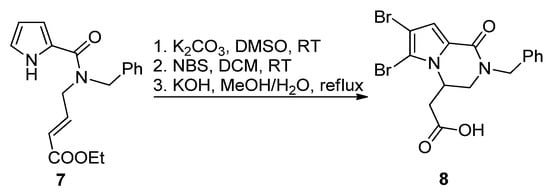
The most common way toward pyrrolopyrazinones is fusing a pyrazinone to a pyrrole. One way to realize this is starting from
1H
-pyrrole-2-carboxamide bearing electrophilic groups on the amide that react in an intramolecular fashion with the nucleophilic pyrrole nitrogen. Several electrophilic groups are possible. Electron-poor alkenes can undergo aza-Michael addition, as in the base-catalyzed formation of
N
-benzyl longamide B derivative
8
from the corresponding open chain pyrrole-2-amide
7
after potassium carbonate (K
2
CO
3
)-catalyzed cyclization, bromination with
N
-bromosuccinimide (NBS) and saponification (
Scheme 1) [24]. A similar aza-Michael cyclization was reported in the total synthesis of longamide B and cyclooroidin via the Wadsworth–Horner–Emmons olefination of longamide A [25][26] or in the 1,8-diazabicyclo[5.4.0]undec-7-ene(DBU)-catalyzed cyclization of precursors to kinase inhibitors
) [32]. A similar aza-Michael cyclization was reported in the total synthesis of longamide B and cyclooroidin via the Wadsworth–Horner–Emmons olefination of longamide A [33,34] or in the 1,8-diazabicyclo[5.4.0]undec-7-ene(DBU)-catalyzed cyclization of precursors to kinase inhibitors 5 [27]. An enantioselective aza-Michael cyclization (up to 56%ee) was realized with compounds analogous to
[29]. An enantioselective aza-Michael cyclization (up to 56%ee) was realized with compounds analogous to 7
in the presence of a chiral
N-benzylammonium phase transfer catalyst derived from quinine [28].
-benzylammonium phase transfer catalyst derived from quinine [35].
Scheme 1.
Aza-Michael reaction leading to a longamide B derivative.
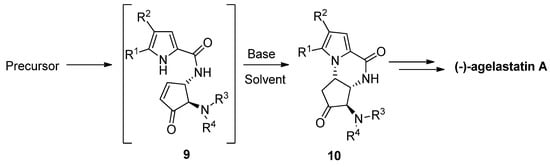
The total synthesis of (-)-agelastatin A involved a similar aza-Michael reaction to an enone intermediate
9, which was generated by the oxidation of an allylic alcohol precursor [29] or by a metathesis reaction [30]. Different bases were tried for the cyclization of
, which was generated by the oxidation of an allylic alcohol precursor [36] or by a metathesis reaction [37]. Different bases were tried for the cyclization of 9
to the intermediate
10
that then could be further elaborated to the natural product. It was found that diisopropylethylamine (DIPEA) in THF is a suitable base/solvent combination after the acidity of the pyrrole is increased by bromination, whereas nonbrominated pyrrole
9 resulted in the recovery of the starting material, rearrangement and/or decomposition [29][31][32]. Many variants of this cyclization have been described, with other base/solvent combinations like cesium carbonate in methanol [30] or THF [33] at room temperature, potassium carbonate in dimethyl sulfoxide (DMSO) at 100 °C [34], trimethylamine in acetonitrile (ACN) at −20 °C [35] and triethylamine (Et
resulted in the recovery of the starting material, rearrangement and/or decomposition [36,38,39]. Many variants of this cyclization have been described, with other base/solvent combinations like cesium carbonate in methanol [37] or THF [40] at room temperature, potassium carbonate in dimethyl sulfoxide (DMSO) at 100 °C [41], trimethylamine in acetonitrile (ACN) at −20 °C [42] and triethylamine (Et 3
N) in DMSO at room temperature with the in situ generation of enone
9 by the elimination of a sulfone group [36][37] (
by the elimination of a sulfone group [43,44] ( Scheme 2
).
Scheme 2.
Aza Michael reaction as part of (-)-agelastatin total synthesis.
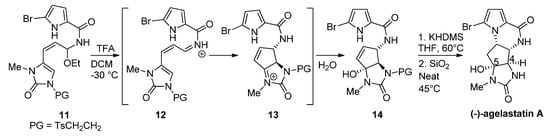
Instead of changing the nucleophilicity of the pyrrole, the electrophilicity of the double bond may be increased by the addition of a Brønsted or Lewis acid. In fact, the biosynthesis of hanishin or longamide B has been described as involving the protonation of a precursor analogous to
7
by an appropriate enzyme [7]. In a bioinspired total synthesis of rac
-agelastatin A, a cascade process occurs starting from a hemiaminal
11
that is converted with trifluoroacetic acid (TFA) into a reactive iminium salt
12
that cyclizes to intermediate
13
and then undergoes the addition of water to give the hydroxyl derivative
14
. The deprotection of
14
and cyclization by heating in the presence of silica (SiO
2) at 45 °C affords agelastatin A (68%) and a minor amount (13%) of its 4,5-epimer [38] (
) at 45 °C affords agelastatin A (68%) and a minor amount (13%) of its 4,5-epimer [45] ( Scheme 3
). We can also mention a similar report wherein trifluoroethanol functions as an acidic medium (40 °C) for the diastereoselective cyclization of
14 to agelastatin A [39].
Rac-cyclooroidin has been prepared in excellent yield (93%), by heating the formic acid salt of the acyclic precursor at 95 °C for 45 h in a sealed tube [40].
-cyclooroidin has been prepared in excellent yield (93%), by heating the formic acid salt of the acyclic precursor at 95 °C for 45 h in a sealed tube [47].
Scheme 3.
Silica-promoted synthesis of (-)-agelastatin A.
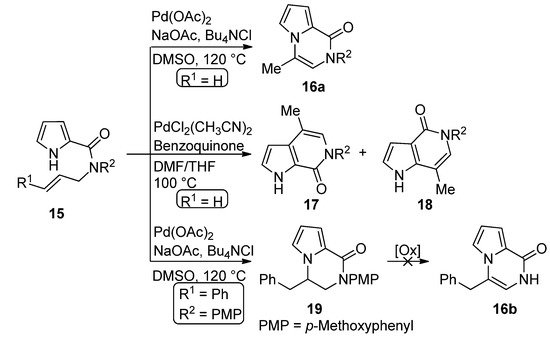
The palladium-catalyzed cyclization of
N
-allyl pyrrole-2-carboxamide
15
(R
1
= H) leads to different products depending on the catalyst. In the presence of palladium acetate (0.1 eq), sodium acetate and tetrabutylammonium chloride (Bu
4
NCl) in DMSO at 120 °C, the pyrrolo[1,2-
a
]pyrazine
16a
is formed. On the other hand, PdCl
2
(CH
3
CN)
2
catalyst (0.1 eq.) in a dimethylformamide (DMF)/tetrahydrofuran(THF) mixture at 100 °C, in the presence of a stoichiometric benzoquinone oxidant, gave a 1:1 mixture of the two isomeric [2,3-
c
] and [3,2-
c
] fused pyrrolopyridinone derivatives
17
and
18, apparently as the result of cyclization involving the 2-position of the pyrrole followed by rearrangement [41]. Remarkably, when the Pd(OAc)
, apparently as the result of cyclization involving the 2-position of the pyrrole followed by rearrangement [48]. Remarkably, when the Pd(OAc) 2
method was applied to the
N
-cinnamyl derivative
15
(R
1
= Ph), the dihydro derivative
19
was obtained in modest yield and different oxidants failed to afford the corresponding pyrrolo[1,2-
a
]pyrazine
16b [42] (
Scheme 4
).
Scheme 4.
Palladium-catalyzed cyclization of
N
-allyl pyrrole-2-carboxamide.
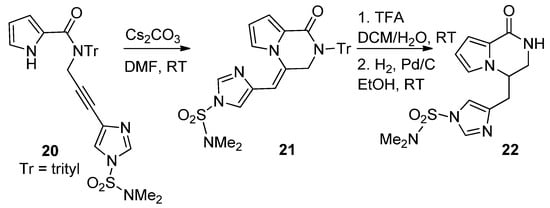
The cyclization reactions of pyrrole nitrogen onto alkyne substituents were studied in basic circumstances. Thus,
N
-imidazolylpropargyl-substituted pyrrole-2-carboxamide
20
was favorably converted to the pyrrolopyridazinone
21
, by an 6-
exo
-dig process, using cesium carbonate (Cs
2
CO
3
) in DMF at room temperature. Further deprotection and
exo
-double bond reduction yielded cyclooroidin analog
22 [43] (
Scheme 5
).
Scheme 5.
Base-catalyzed ring closure of
N
-propargyl pyrrole-carboxamides.
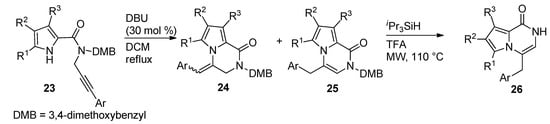
Other examples of the base-catalyzed ring closure of
N-propargyl derivatives were reported, with 30 mol% DBU in dichloromethane (DCM) at reflux temperature [44], which led to a mixture of pyrrolopyrazinone isomers
-propargyl derivatives were reported, with 30 mol% DBU in dichloromethane (DCM) at reflux temperature [51], which led to a mixture of pyrrolopyrazinone isomers 24
and
25
with an
exo
double bond and an
endo
double bond, respectively. The isomerization of the
exo
-isomers
24
to the thermodynamically preferred
endo
-product
25
and the deprotection mediated by triisopropylsilane (
i
Pr
3
SiH) and TFA under microwave (MW) heating, only yielded
26
(
Scheme 6
).
Scheme 6.
Ring closure of
N
-(3-arylpropargyl) pyrrole-carboxamides.
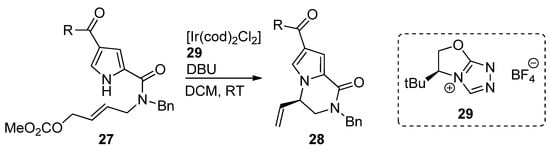
An iridium (I) complex with chiral
N
-heterocyclic carbene ligand
29
was used as a catalyst for the intramolecular aminoallylation of acylpyrroles
27
, leading to (
R
)-vinyl-substituted pyrrolopyrazinones
28 [45] in e.e. of 92–95% (
Scheme 7
). In a subsequent report, an Ir/phosphoramidite catalytic system was explored to obtain the (
S)-isomer, which is used as a starting material for the total synthesis of longamide B, hanishin or cyclooroidin analogs [46].
)-isomer, which is used as a starting material for the total synthesis of longamide B, hanishin or cyclooroidin analogs [53].
Scheme 7.
Iridium-catalyzed intramolecular allylation strategy toward pyrrolopyrazinones.
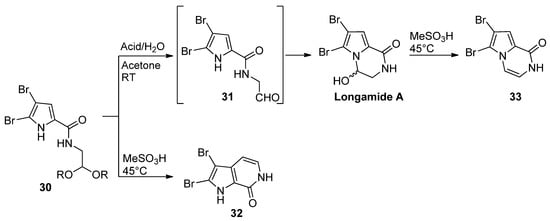
Pyrrole-2-carboxamides
30
N
-substituted with an acetal-protected aldehyde function cyclized upon acid-catalyzed deprotection. The outcome of the reaction is dependent on the reaction conditions. The treatment of
30
with 4-toluenesulfonic acid or HCl in acetone/water at room temperature gave longamide A, probably after the cyclization of the intermediate aldehyde
31. Racemic longamide A can be separated into the two enantiomers through chiral chromatography, but these racemize at room temperature within minutes [47]. On the other hand, the isomeric pyrrolopyridine
. Racemic longamide A can be separated into the two enantiomers through chiral chromatography, but these racemize at room temperature within minutes [54]. On the other hand, the isomeric pyrrolopyridine 32
was formed on the heating of
30
with methanesulfonic acid (MeSO
3
H).
Longamide A
was formed on heating with methanesulfonic acid or on treatment with 4-toluenesulfonyl chloride, and trimethylamine gave the dehydrated pyrazinone
33 [48] (
Scheme 8
). Unprotected ketone analogs of
31 (with different degrees of bromination on the pyrrole ring) were shown to be in equilibrium with the hydroxypyrrolopyrazinones, but the oxidation of the pyrrole ring to a 2-hydroxypyrrolin-5-one with Selectfluor gave the ring-opened product [49].
(with different degrees of bromination on the pyrrole ring) were shown to be in equilibrium with the hydroxypyrrolopyrazinones, but the oxidation of the pyrrole ring to a 2-hydroxypyrrolin-5-one with Selectfluor gave the ring-opened product [56].
Scheme 8.
Alternate intramolecular reactions of acetals and pyrrole.
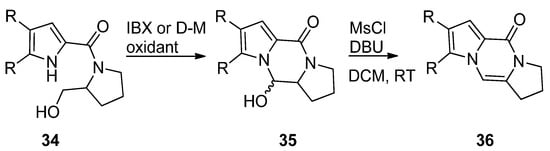
The pyrrole-2-carbamide
34 (R = H) derived from prolinol on oxidation with 2-iodoxybenzoic acid (IBX) in DMSO at room temperature [50] or Dess–Martin (D-M) reagent in DCM at room temperature [51] gave the hydroxypyrrolopyrazinone
(R = H) derived from prolinol on oxidation with 2-iodoxybenzoic acid (IBX) in DMSO at room temperature [57] or Dess–Martin (D-M) reagent in DCM at room temperature [58] gave the hydroxypyrrolopyrazinone 35
, which could be dehydrogenated with phosphoryl chloride (POCl
3) in pyridine at room temperature [50] or with mesyl chloride and DBU in DCM at room temperature [51] to afford the tricyclic compound
) in pyridine at room temperature [57] or with mesyl chloride and DBU in DCM at room temperature [58] to afford the tricyclic compound 36
(
Scheme 9
). The compounds
35
and
36 (R = H, Br) were also obtained in a similar sequence from the reduction of the pyrrolecarboxamide connected to the Weinreb amide of proline with lithiumaluminium hydride [52] or with hydroxyprolinate (diisobutylaluminum hydride reduction) [53], in the framework of total syntheses of dibromophakellstatin.
(R = H, Br) were also obtained in a similar sequence from the reduction of the pyrrolecarboxamide connected to the Weinreb amide of proline with lithiumaluminium hydride [59] or with hydroxyprolinate (diisobutylaluminum hydride reduction) [60], in the framework of total syntheses of dibromophakellstatin.
Scheme 9.
Cyclization of prolinol derivatives.
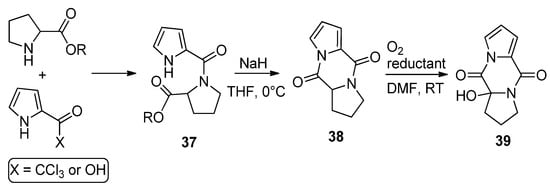
Amides
37
, resulting from the condensation of 2-trichloroacetylpyrrole (or pyrrole-2-carboxylic acid and amidation reagents) and different amino esters derived from natural amino acids (shown here for proline), were cyclized with sodium hydride in THF to the diketopiperazine derivatives
38 in high yield. Several reports have appeared in the literature [52][54][55][56][57]. These compounds
in high yield. Several reports have appeared in the literature [59,61,62,63,64]. These compounds 38
could be oxygenated with molecular oxygen to a hydroperoxide and could be reduced in situ with dibutyl sulfide ((
n
-Bu)
2
S) or triphenyl phosphine (PPh
3
), affording the hydroxy product
39 in high yield [55] (
Scheme 10
). Recently, it was found that these diketopiperazines
38 could function as catalysts in oxygenation reactions [58], and the oxygenation of compound
could function as catalysts in oxygenation reactions [65], and the oxygenation of compound 38 in the presence of guanidine has also been mentioned as acting in the biogenesis of 2-aminoimidazolidinone metabolites from sponges [54].
in the presence of guanidine has also been mentioned as acting in the biogenesis of 2-aminoimidazolidinone metabolites from sponges [61].
Scheme 10.
Diketopiperazine derivatives from amino esters and pyrrole-2-carboxylic reagents.
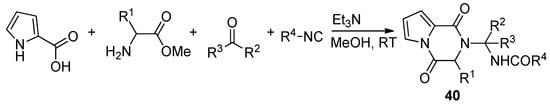
Pyrrole-2-carboxylic acid, carbonyl compounds, isocyanides and amino esters undergo the four-component Ugi reaction to afford the adducts, which cyclized spontaneously at room temperature in methanol and triethylamine (Et
3N) to afford a library of polysubstituted pyrrole diketopyrazines 40 [59] (
N) to afford a library of polysubstituted pyrrole diketopyrazines 40 [66] ( Scheme 11). An extensive discussion of pyrrolo-fused diketopiperazines is out of the scope of this review. Instead, we give a few additional key references [50][58][60][61][62][63].
). An extensive discussion of pyrrolo-fused diketopiperazines is out of the scope of this review. Instead, we give a few additional key references [57,65,67,68,69,70].
Scheme 11.
Pyrrolopyrazinones by Ugi four component reaction.
2.2. Starting from 1-Monosubstituted Pyrroles
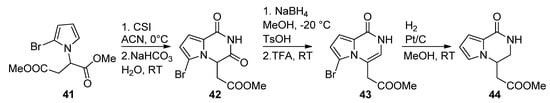
There are few examples in the literature of a 1-monosubstitued pyrrole that is converted to a pyrrolopyrazinone. Thus, the pyrrole
41
was prepared from aspartic acid dimethyl ester and reacted with chlorosulfonyl isocyanate (CSI), affording the pyrrolopyrazinedione
42
. Reduction with sodium borohydride in methanol, and dehydration, gave the pyrazinone
43
, which was then reduced with Pt/C and H
2
, simultaneously removing the bromine, to longamide B analogs
44 [64] (
Scheme 12
). Compounds analogous to
42
have also been prepared from the 2-trichloracetylation of
41 followed by substitution with primary amines [20][63].
followed by substitution with primary amines [20,70].
Scheme 12.
Cyclization of 1-monosubstituted pyrrole with CSI.
2.3. Starting from 1,2-Disubstituted Pyrroles
There are a number of reports wherein a 1,2-disubstituted pyrrole was used as a starting material for the formation of a pyrrolopyrazinone. This may be done with (1) a single acyclic precursor containing an electrophilic carbonyl group at the 2-position and a nucleophilic substituent (mostly amine) at the 1-position, or (2) vice versa, or (3) the condensation of two components of which one contains the disubstituted pyrrole.
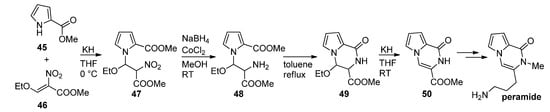
Thus, methyl 2-pyrrolecarboxylate
45
was combined with a nitroalkene
46
in the presence of potassium hydroxide to give a nitroalkyl-substituted pyrrole
47
, which was then reduced with sodium borohydride (NaBH
4
)/cobalt(II)chloride (CoCl
2
), and the amine
48
cyclized at reflux temperature in toluene after which ethanol was eliminated from
49
in basic medium, leading to the product
50 that was used as a starting material for the first total synthesis of peramide [23][65] (
that was used as a starting material for the first total synthesis of peramide [23,72] ( Scheme 13
). As an alternative to a nitro compound, a
N
-CH
2CN functionality can be introduced, using iodoacetonitrile, which can be reduced to the amine, which then further cyclizes to a pyrrolopyrazinone [66].
CN functionality can be introduced, using iodoacetonitrile, which can be reduced to the amine, which then further cyclizes to a pyrrolopyrazinone [27].
Scheme 13.
First total synthesis of peramide.
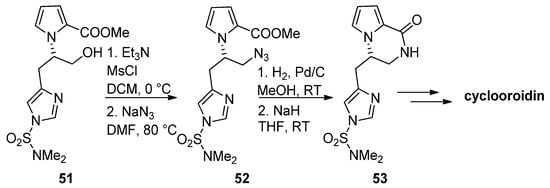
The azide function is a common precursor for amine that can easily be generated in situ by catalytic reduction. Therefore, in the framework of a total synthesis of cyclooroidin, alcohol
51
was mesylated and converted into azide
52
, and catalytic hydrogenation followed by the addition of sodium hydride (NaH) resulted in the formation of the pyrrolopyrazinone
53, which was then further elaborated to cyclooroidin [67] (
, which was then further elaborated to cyclooroidin [73] ( Scheme 14). Similar strategies have been used in the total synthesis of (-)-hanishin [68] or in the synthesis of histone deacetylase inhibitors [69] and the inhibitors of the mycobacterium ATP synthase [70].
). Similar strategies have been used in the total synthesis of (-)-hanishin [74] or in the synthesis of histone deacetylase inhibitors [75] and the inhibitors of the mycobacterium ATP synthase [76].
Scheme 14.
Azides as intermediates in the total synthesis of cyclooroidin.
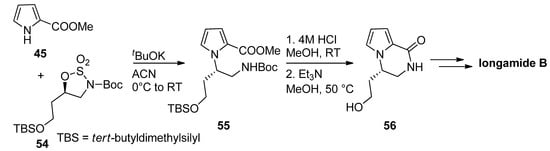
Typical amine-protecting groups like
tert
-butoxycarbonyl (Boc) and fluorenylmethoxycarbonyl (Fmoc) can also be used in intermediates leading to pyrrolopyrazinones. Thus, the condensation of methyl 2-pyrrolocarboxylate
45
with cyclic sulfamidates
54
and the potassium
tert
-butoxide base gave the precursor
55
, which was deprotected with acid and then cyclized, mediated by triethylamine (Et
3N) [71]. The resulting pyrrolopyrazinone
N) [77]. The resulting pyrrolopyrazinone 56 can then be further elaborated to longamide B or hanishin [72][71][73] (
can then be further elaborated to longamide B or hanishin [30,77,78] ( Scheme 15). Further examples of this strategy have been reported toward longamide B derivative, kinase inhibitors [74] and mGluR1 antagonists [66], and we can also mention a Fmoc-based total synthesis of cyclooroidin [75].
). Further examples of this strategy have been reported toward longamide B derivative, kinase inhibitors [28] and mGluR1 antagonists [27], and we can also mention a Fmoc-based total synthesis of cyclooroidin [79].
Scheme 15.
Boc-strategy toward longamide B derivatives.
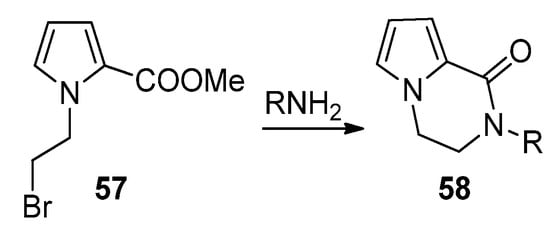
An effective example of a two-component reaction leading to the pyrrolopyrazinone scaffold is the reaction of
N
-(2-bromoethyl)pyrrole-2-carboxylates
57
with amines, leading to the
N
-substituted bicyclic derivatives
58
(
Scheme 16). Probably the reaction starts with the substitution of the bromine by the amine, followed by lactamization. Several examples were reported [76][66][77]. In the framework of agelastatin total synthesis, some examples were reported where, in the presence of sodium hydride, an amide substituted a bromine at the side chain connected to nitrogen, proving that the opposite order of reactions is also possible [78][79].
). Probably the reaction starts with the substitution of the bromine by the amine, followed by lactamization. Several examples were reported [25,27,80]. In the framework of agelastatin total synthesis, some examples were reported where, in the presence of sodium hydride, an amide substituted a bromine at the side chain connected to nitrogen, proving that the opposite order of reactions is also possible [81,82].
Scheme 16.
Bromine substitution and lactamization with primary amines.
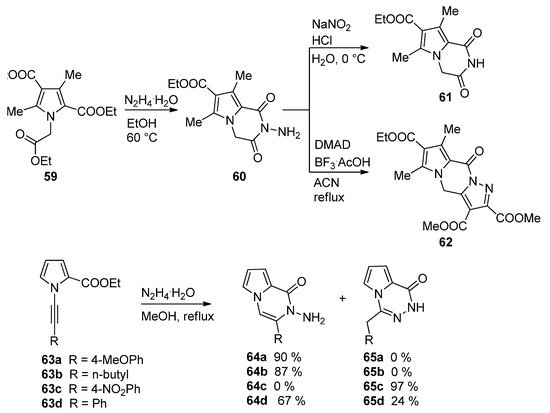
The condensation of hydrazine in ethanol with the triester
59
led to the
N
-aminopyrrolopyrazinone
60
, which, upon treatment with sodium nitrite and acid, gave the deaminated derivative
61
, and the condensation of
60
with dimethyl acetylenedicarboxylate (DMAD) catalyzed by BF
3
/acetic acid (BF
3
·AcOH) complex in acetonitrile afforded the interesting pyrazolo-fused analog
62 [80]. A related cyclization of 1-alkynylpyrrole-2-carboxylate
[83]. A related cyclization of 1-alkynylpyrrole-2-carboxylate 63
and hydrazine hydrate occurred with remarkable selectivity. Electron-rich aryl groups or alkyl groups R give the pyrrolopyrazinone
64a,b
, whereas for R = 4-nitrophenyl, only the 1,2,4-triazine
65c
is obtained. The phenyl substituted analogs
63d
gave a mixture of the two products
64d
and
65d [81] (
Scheme 17
).
Scheme 17.
Reactions of biselectrophilic pyrroles with hydrazine.
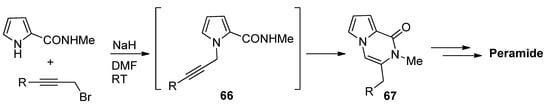
N
-Propargylpyrrole-2-carboxamides
66
prepared in situ were cyclized to pyrrolopyrazinones
67 using NaH in DMF at room temperature [82], which was applied to a total synthesis of peramide [83] (
using NaH in DMF at room temperature [85], which was applied to a total synthesis of peramide [86] ( Scheme 18
).
Scheme 18.
N-
propargylpyrrole-2-carboxamide synthesis and cyclization.
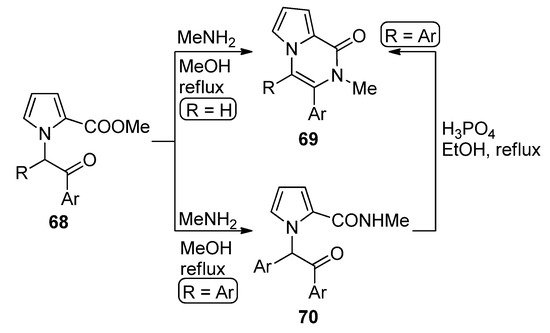
N
-(phenacyl)substituted pyrrole-2-carboxylates
68
(R = H) reacted with methylamine (MeNH
2
) in methanol at reflux to give direct access to pyrrolopyrazinones
69
. The diaryl-substituted analogs
68
(R = Ar), on the other hand, gave the amidation product
70
, which could be converted to the diaryl analog of
69
(R = Ar) by heating
70 at reflux in a 85% phosphoric acid/ethanol mixture [84] (
at reflux in a 85% phosphoric acid/ethanol mixture [87] ( Scheme 19
). An early study of the synthesis of analogs of
69
involved the condensation of amines with intermediate pyrrolo-1,4-oxazines (derived from the
N-alkylation of 2-(trichloroacetyl)pyrrole with chloroacetone) [85]. When acetal-protected 1-acetaldehyde 2-carboxamidepyrrole is deprotected in reflux acetic acid, the unsubstituted derivative of pyrrolopyrazinone analog of
-alkylation of 2-(trichloroacetyl)pyrrole with chloroacetone) [88]. When acetal-protected 1-acetaldehyde 2-carboxamidepyrrole is deprotected in reflux acetic acid, the unsubstituted derivative of pyrrolopyrazinone analog of 69 was obtained [86].
Scheme 19.
Cyclization of
N
-phenacylpyrroles and methylamine.
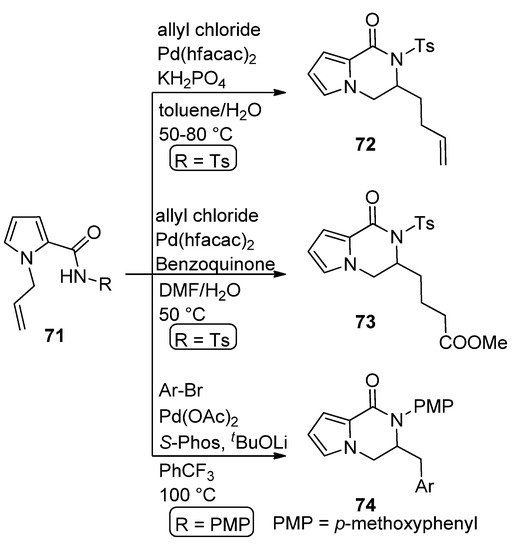
The carboamination of
N
-allyl pyrrole carboxamide
71
(R = Ts) with allyl chloride in the presence of 10 mol% Pd(II) hexafluoroacetoacetate (Pd(hfacac)
2
) and potassium dihydrogenphosphate in toluene/water at 50–80 °C leads to dihydropyrrolopyrazinone
72 [87]. A similar reaction carried out with the same catalyst in the presence of benzoquinone in DMF/water gave the oxygenated analog
[90]. A similar reaction carried out with the same catalyst in the presence of benzoquinone in DMF/water gave the oxygenated analog 73 [88]. Similarly, the carboamination of
[91]. Similarly, the carboamination of 71
(R =
p
-methoxyphenyl, PMP) with aryl bromides in the presence of Pd(OAc)
2
/
S
-Phos catalyst at 100 °C afforded the dihydropyrrolopyrazinone
74 [89] (
Scheme 20
).
Scheme 20.
Pd(II)-catalyzed carboamination reactions.
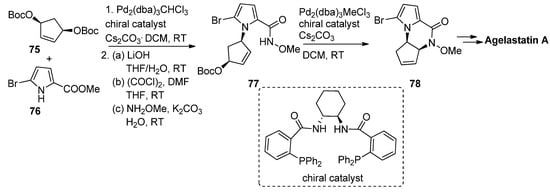
Allylpalladium species can function as electrophiles in cyclization reactions leading to pyrrolopyrazinones, and this has mainly been used in the context of the total synthesis of natural products. Thus, an enantioselective synthesis of agelastatin A reported by Trost et al. involved firstly the palladium-catalyzed allylation starting from the prochiral bisprotected cyclopentenediol
75
with 5-bromopyrrolecarboxylate
76
in the presence of a chelating chiral bisphosphine catalyst, affording the precursor that then, after conversion to the
N
-methoxyamide
77
, underwent a second intramolecular allylation to afford pyrrolopyrazinone
78, which could then be further elaborated to agelastatin A [90] (
, which could then be further elaborated to agelastatin A [93] ( Scheme 21). Many variants on this allylation strategy, mostly as a part of agelastatin natural product total syntheses, were reported [91][92][93][94][95][96].
). Many variants on this allylation strategy, mostly as a part of agelastatin natural product total syntheses, were reported [94,95,96,97,98,99].
Scheme 21.
Palladium-catalyzed intramolecular allylation strategy toward pyrrolopyrazinone.
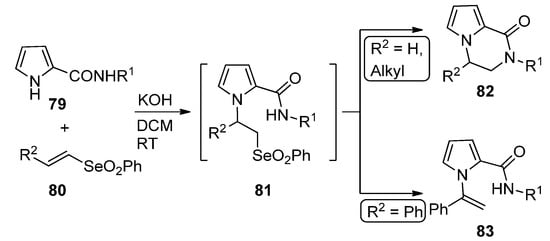
A remarkable domino reaction of pyrrole-2-carboxamides
79
and vinyl selenones
80
(R
2
= H, alkyl) in basic medium occurs via an initial Michael addition, followed by the intramolecular substitution of intermediate
81
, leading to pyrrolopyrazinone
82
. In the case of styryl selenone
80
(R
2
= Ph), the
N
-(1-phenylethenyl)pyrrole
83 is formed instead [97] (
is formed instead [100] ( Scheme 22
).
Scheme 22.
Domino reaction of pyrrole-2-carboxamides and vinyl selenones.
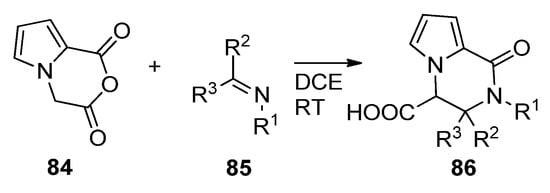
The Castagnoli–Cushman reaction (CCR) is a ring opening/ring closure reaction of cyclic anhydrides with imines. When applied to anhydride
84
, prepared from the diacid with trifluoroacetic anhydride, condensation with different imines
85
in 1,2-dichloroethane (DCE) at room temperature led to a large variety of trisubstituted pyrrolopyrazinones
86 [98] (
Scheme 23
). The reaction has also been applied to substituted pyrrole anhydrides
84 [99].
Scheme 23.
Castagnoli–Cushman reaction of pyrrole cyclic anhydrides.
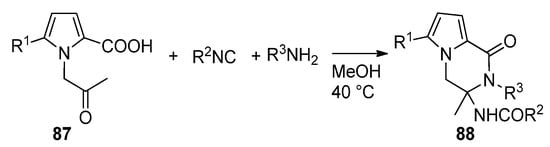
The multicomponent Ugi reaction has been applied to
N
-(2-oxopropyl)pyrrole-2-carboxylic acids
87
. In this case, two of the four components (the acid and the ketone) of the Ugi reaction are present on the pyrrole moiety, and two more are added under the form of an isonitrile and an amine. This leads to a library of polysubstituted pyrrolopyrazinones
88 [100] (
Scheme 24). Compounds of this type have been described as dengue inhibitors [101].
). Compounds of this type have been described as dengue inhibitors [104].
Scheme 24.
Ugi reaction toward pyrrolopyrazinones.
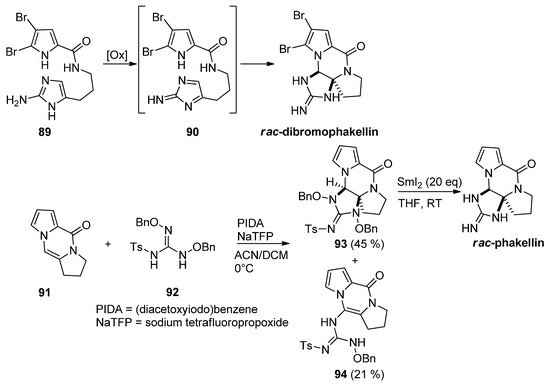
Different approaches to
rac
-dibromophakellin or other tetracyclic marine natural products rely on the intramolecular cycloaddition of a reactive intermediate
90
generated after the oxidation of aminoimidazole
89. This chemistry has been reviewed before [102]. Recently, an intermolecular variant has been described starting from tricyclic
. This chemistry has been reviewed before [105]. Recently, an intermolecular variant has been described starting from tricyclic 91
, which was reacted with guanidine derivative
92
after oxidation with (diacetoxyiodo)benzene (PIDA) and sodium tetrafluoropropoxide (NaTFP) base. Fair amounts of cycloadduct
93
were obtained together with a minor amount of open chain compound
94
. The reduction of
93
with an excess of SmI
2
then gave the
rac-phakellin [103] (
Scheme 25
). Other approaches involving regio- and stereoselective additions of nitrogen species to analogs of
91 have been mentioned in the framework of dibromophakellstatin total syntheses [51][102][104][105][106].
have been mentioned in the framework of dibromophakellstatin total syntheses [58,105,107,108,109].
Scheme 25.
Oxidative additions of aminoimidazoles and guanidine derivatives toward phakellin natural products.
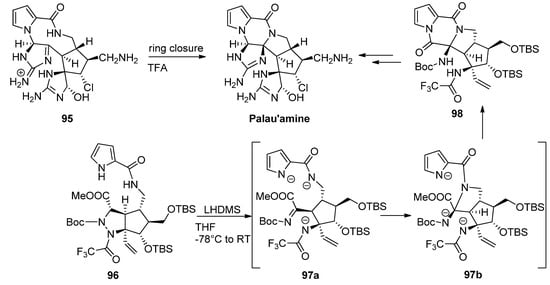
Different strategies for the total synthesis of palau’amine have been reported, and an exhaustive discussion is beyond the scope of this text, so the reader is referred to some dedicated reviews [107]. One of methods that stand out is the ring contraction of the macrocycle
Different strategies for the total synthesis of palau’amine have been reported, and an exhaustive discussion is beyond the scope of this text, so the reader is referred to some dedicated reviews [110]. One of methods that stand out is the ring contraction of the macrocycle 95 reported by Baran as the final step toward palau’amine [108]. One other remarkable process is a cascade reaction of precursor
reported by Baran as the final step toward palau’amine [111]. One other remarkable process is a cascade reaction of precursor 96
with the initial deprotonation and ring opening of the tetrahydropyrazole toward intermediate
97a
followed by formation or the pyrrolidine ring of intermediate
97b
and subsequent formation of the diketopiperazine
98, which then was further elaborated to palau’amine [109] (
, which then was further elaborated to palau’amine [112] ( Scheme 26
).
Scheme 26.
Different cyclization strategies to palau’amine.
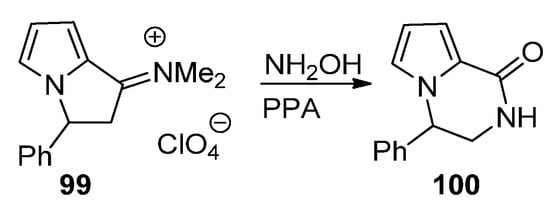
A final strategy toward pyrrolopyrazinones starting from pyrrole building blocks is through the ring expansion of pyrrolizidine derivatives, using the Beckmann rearrangement of the phenyl derivative
99
, and, after condensation with hydroxylamine and heating in polyphosphoric acid (PPA), the pyrrolopyrazine
100 is obtained [110] (
Scheme 27
).
Scheme 27.
Ring expansion of pyrrolizidine to pyrrolopyrazinone.
2.4. Fusion of a Pyrrole to a Pyrazinone Derivative
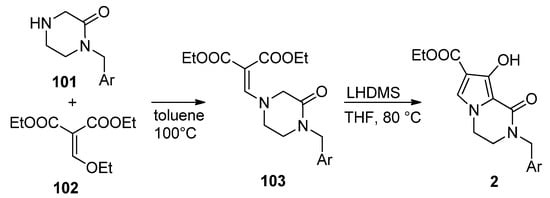
This approach has been much less studied than the pyrrole-first method, with only a few reports so far. Thus, the integrase inhibitors
2
were obtained, starting from pyrazinone
101
and diethyl ethoxymethylene malonate
102
, by heating at 100 °C in toluene. The resulting enamine
103
was then cyclized with lithium bis(trimethylsilyl)amide LHMDS at 80 °C in THF, affording compound
2 [111] (
Scheme 28
).
Scheme 28.
Synthesis of hydroxy-substituted pyrrolopyrazinones.
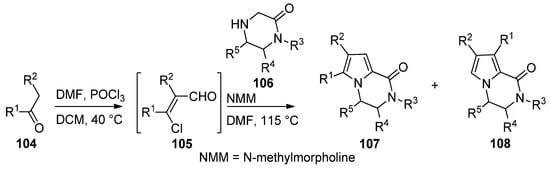
An efficient two step synthesis of polysubstituted pyrrolopyrazinones started with the Vilsmeier–Haack chloroformylation of readily available ketones
104
to afford biselectrophilic 2-chloroacrolein intermediate
105
, which was then condensed with pyrazinones
106
in the presence of
N
-methylmorpholine (NMM) base in DMF at 115 °C, affording compounds
107
in fair yields, in some case accompanied with the isomer
108 [112] (
Scheme 29
).
Scheme 29.
Two-step synthesis of pyrrolopyrazinones from 2-chloroacroleins.
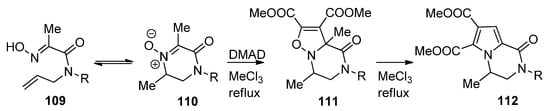
Isoxazolino-fused piperazinones
111
were prepared via the 1,3-dipolar cycloaddition of nitrones
110
, which was in equilibrium with the open chain oximes
109
, to dimethyl acetylene dicarboxylate (DMAD). Remarkably, upon heating a rearrangement occurs to pyrrolopyrazinones
112, presumably via a multistep ring contraction/ring expansion mechanism [113] (
, presumably via a multistep ring contraction/ring expansion mechanism [115] ( Scheme 30
).
Scheme 30.
Synthesis and rearrangement of isoxazolinopyrazinones.
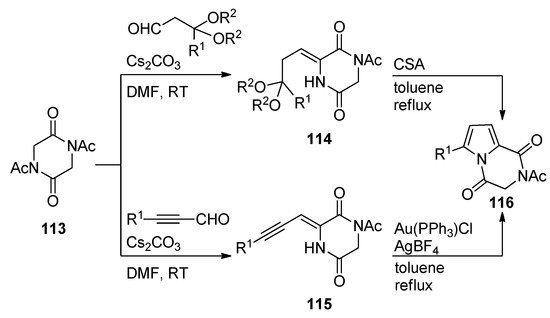
Diketopiperazines
113
underwent base-catalyzed aldol condensations with different aldehydes, affording adducts that, in the case of acetal substituents, as for
114
, underwent camphorsulfonic acid (CSA)-mediated cyclization on heating in toluene to pyrrolodiketopiperazines
116
. The aldol condensation products
115
resulting from alkynyl aldehydes underwent gold-catalyzed cyclization under similar conditions, giving an alternative preparation for compounds
116
with a larger scope of R
1-substituents [114] (
Scheme 31
).
Scheme 31.
Brønsted and Lewis acid-catalyzed cyclization reactions of diketopiperazines.
2.5. Multicomponent Reactions
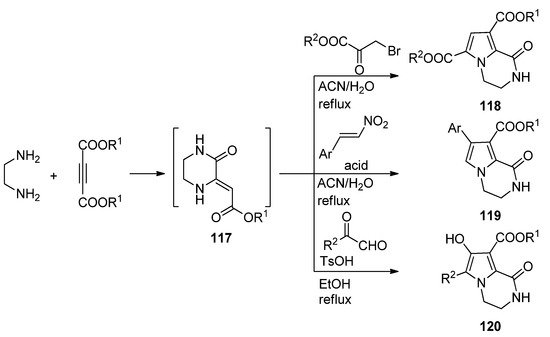
A three-component reaction of 1,2-diaminoethane, dialkyl acetylene dicarboxylate and different biselectrophiles present a very straightforward way to pyrrolopyrazinones. Probably the diamine first reacts with the electrophilic acetylene, and the intermediate pyrazinone derivative
117
then cyclizes with the biselectrophile. Thus, reaction with bromopyruvate in acetonitrile or water at reflux resulted in diester
118 [115][116]. On the other hand, reactions with nitrostyrene, catalyzed by sulfamic acid (SA) in acetonitrile [117] or Fe
[117,118]. On the other hand, reactions with nitrostyrene, catalyzed by sulfamic acid (SA) in acetonitrile [119] or Fe 3
O
4
@SiO
2
-OSO
3H magnetic nanoparticles in water [33] afforded aryl derivatives
H magnetic nanoparticles in water [40] afforded aryl derivatives 119
, and condensation with methyl- or arylglyoxal in ethanol at reflux with
p
-toluenesulfonic acid (TsOH) catalysis gave hydroxyl derivatives
120 [118] (
Scheme 32
).
Scheme 32.
Three-component reactions leading to dihydropyrrolopyrazinones.
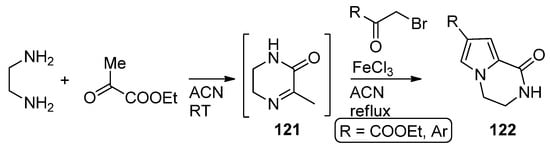
In a variant of this three component reaction, 1,2-diaminoethane and ethyl pyruvate are combined at room temperature in acetonitrile, and then
α
-bromo ketones are added and the mixture heated in the presence of iron (III) chloride to afford
122
via the reaction of intermediate pyrazine
121 with the bromoketone [119] (
with the bromoketone [121] ( Scheme 33
).
Scheme 33.
Three-component reaction with ethyl pyruvate.
2.6. Miscellaneous
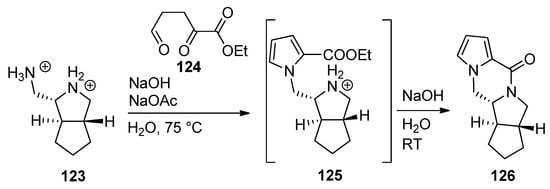
The ABDE core of palau’amine was constructed from the dibromide salt of diamine
123
and triscarbonyl compound
124
by a cascade reaction involving a Paal–Knorr pyrrole synthesis, leading to intermediate pyrrole
125
, which, after neutralization, undergoes subsequent lactamization to afford tetracyclic
126 [120] (
Scheme 34
).
Scheme 34.
Cascade process to the ABDE core of palau’amine.
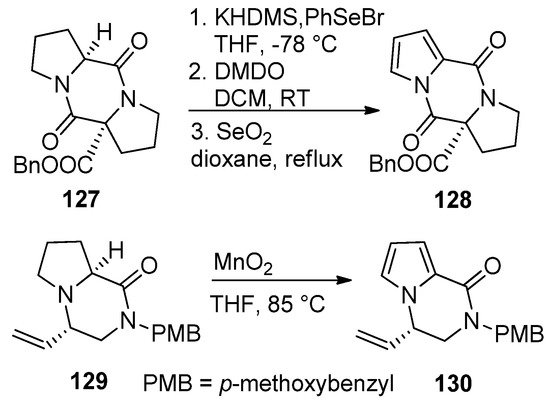
Pyrrolidino-fused diketopiperazines
127
could be oxidized to the pyrrolodiketopiperazines
128
after sequential deprotonation, phenylselenation, oxidation with dimethyldioxirane (DMDO)/elimination and aromatization of the intermediate pyrroline by heating with selenium dioxide in dioxane. Earlier attempts to aromatize
127 with 2,3-dichloro-5,6-dicyano-1,4-benzoquinone (DDQ) gave a lower yield (20–30%) and was accompanied by difficult purification [104]. Recently, the proline-derived compound
with 2,3-dichloro-5,6-dicyano-1,4-benzoquinone (DDQ) gave a lower yield (20–30%) and was accompanied by difficult purification [107]. Recently, the proline-derived compound 129
was oxidized to the pyrrole
130
with MnO
2 in THF at 85 °C without effecting the vinyl or dihydropyrazinone parts [121] (
in THF at 85 °C without effecting the vinyl or dihydropyrazinone parts [123] ( Scheme 35
).
Scheme 35.
Aromatization of pyrrolidino-fused diketopiperazines and pyrazinones.
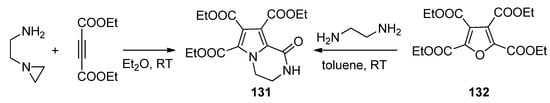
The condensation of
N
-(2-aminoethyl)aziridine with two equivalents of diethyl acetylenedicarboxylate gave the triester
131
, the same compound that could be obtained through the ring transformation of the furan tetraester
132 and 1,2-diaminoethane [122] (
and 1,2-diaminoethane [124] ( Scheme 36
).
Scheme 36.
Formation of pyrrolopyrazinone triesters.