Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 3 by Amina Yu and Version 2 by Amina Yu.
species belonging to the Phaeophyceae and Rhodophceae classes are primarily potent against herpes simplex virus, followed by human immunodeficiency virus and influenza virus.
- macro-algae
- antioxidant
- antiviral
- ulvan
- subcritical water extraction
Introduction
In 2004, the food and agriculture organization (FAO) introduced a taxa classification of macro-algae according to its pigmentation, brown (Phaeophyceae class), red (Rhodophyceae class), and green (Chlorophyceae class). Since then, different macro-algae classes have gained scholars’ attention for their ecological importance of supplying oxygen to the sea and usage in traditional medicine due to their perceived health benefits [1].
More recently, it has been claimed that macro-algae represent about 9% of biomedical compounds obtained from the sea [2]. Scholars explain that bioactive compounds of macro-algae such as polysaccharides have proven to have an effective antioxidant and antiviral activity. They argue that those polysaccharides have been developed as a chemical defense mechanism to the harsh environments in which they grow, such as variation in salinity, solar radiation and tidal waves, competition for space and nutrients [3,4][3][4].
Therefore, recent research argues that marine metabolites can shape the future of the bioeconomy [5] and might emerge as a new wave of promising drugs [6]. However, despite this claim, only a few studies have provided a systematic literature review on their antioxidant and antiviral activity along with their optimal extraction method. Therefore, this study fills a gap in the current literature by illustrating a comparative review of the antioxidant and antiviral activities of different macro-algae classes and identifying the active metabolite’s optimal extraction medium. Furthermore, the paper discusses the extraction methods of the active metabolites in macro-algae; specifically, the extraction of ulvan polysaccharide from the Ulva species to optimize their use in medicinal products.
Antiviral Activity of Macroalgae
The antiviral activity of macroalgae has been reported consistently early in the literature, for example, Gerber et al. [7] claimed the antiviral activity of macroalgae against influenza B and mumps virus. Similarly, Witvrouw et al. [8] reported that Aghardhiella tenera and Nothogenia fastigiata species of seaweed have antiviral activity towards human immunodeficiency virus (HIV), herpes simplex virus types 1 and 2 (HSV-1 and HSV-2), and respiratory syncytial virus (RSV). Moreover, Witvrouw and De Clercq [9] confirmed the inhibitory effect against the enveloped viral replication by the complex structures of sulphated polysaccharides in macroalgae. In the same line, authors report that carrageenan has a selective inhibitory effect against the enveloped virus and blocked the transmission of several viruses such as HIV, herpes simplex virus, human cytomegalovirus, and human rhinoviruses [10,11][10][11].
In the following decades, researchers confirmed the algal extract’s virucidal effect [12,13,14][12][13][14]. Scholars also confirmed that the low cytotoxicity, and successful use of antivirals from macroalgae in vaginal therapy had made its production for pharmaceutical use widely accepted. Similarly, Ono et al. [15] confirmed that sulphated polysaccharide extracted from macroalgae has anti-HIV activity and was able to inhibit flaviviruses such as dengue virus. Moreover, several researchers have confirmed the inhibitory effects of sulphated polysaccharides derived from macroalgae on the herpes simplex virus strains [16,17][16][17]. Additionally, Vo and Kim [18] as well as Jiao et al. [19], highlighted the association of sulphated polysaccharides from macroalgae with the antiviral activity. Similarly, Pati et al. [20] confirmed that sulphated polysaccharides such as carrageenan, fucoidans, and sulphated rhamno galactans successfully inhibited the enveloped viruses like HIV, and herbs.
Additionally, Grassauer and Prieschl-Grassauer [21] claimed that marine biomass such as carrageenan sulphated polysaccharide can facilitate the protection from the newly discovered coronavirus disease 2019 (COVID-19) which belongs to a family of enveloped viruses, or at least can be used as coating material for protective supplies such as masks and gloves. The same was confirmed by Zaporozhets et al. [22] who reported that the sulphated polysaccharides extracted from marine algae Saccharina japonica showed a significant antiviral activity against the coronavirus. Thus, a potential antiviral medicine can be developed from macroalgae biomass for augmenting the existing antivirals to combat emerging types and variants of enveloped viruses.
Table 1 provides a comprehensive review of the literature for antiviral activity of different Phaeophyceae along with their active metabolite. The review indicates that species belonging to Phaeophyceae are primarily potent against HSV, followed by HIV and influenza virus.
Table 1. A review of antiviral activity of macroalgae—Phaeophycea.
| Macroalgae Taxa |
Macroalgae Species | Bioactive Metabolites | Antiviral Activity |
Reference |
|---|---|---|---|---|
| Phaeophyceae | Ecklonia cava | Phlorotannin (6,6′-Bieckol, 8,8′-bieckol) |
Against HIV |
| Against HSV |
| [ |
| 39 |
| ] |
Table 3 provides a comprehensive review of the literature for antiviral activity of different Chlorophyceae along with their active metabolite. The data emphasize that species belonging to Chlorophyceae class are recorded by most of the scholars to have antiviral activity against HSV-1 and HSV-2.
Table 3. A review of antiviral activity of macroalgae—Chlorophyceae.
| Macroalgae Taxa |
Macroalgae Species | Bioactive Metabolites | Antiviral Activity | Reference | |||
|---|---|---|---|---|---|---|---|
| Chlorophyceae | Codium fragile | Polysaccharides | [ | 23,[2324]][24] | |||
| Against HSV-2 | [ | 16 | ] | Dictyota caribaea horning & schnetter |
Sulphated Fucans | Against HIV | [25] |
| Ulva sulphated polysaccharides |
Peptides (Hexapeptide) | Against HSV | [49] | Ecklonia cava | Phlorotannin (Phloroglucinol, eckol, 7-Phloroeckol, phlorofucofuroeckol, dieckol) |
Against Influenza |
[26] |
| Caulerpa racemose | Sulphated Polysaccharides | Against HSV-2 | [50] | Grateloupia filicina | |||
| Ulva fasciata | Sulphated polysaccharides | Sulphated PolysaccharidesAgainst HSV | Against Semliki Forest & [27] |
||||
| Vaccinia Viruses | [ | 51 | ] | Grateloupia longifolia | Sulphated polysaccharides | ||
| Codium elongatum | Against HIV | [ | 27] | ||||
| Sulphated Polysaccharides | Against Semliki Forest & | Vaccinia Viruses |
Adenocystis utricularis | Sulphated polysaccharides | Against HSV | [13] | |
| Cystoseira indica | Sulphated polysaccharides | Against HSV | [28] | ||||
| Dictyota mertensii | Sulphated polysaccharides | Against HIV | [29] | ||||
| Fucus vesiculosus | Sulphated polysaccharides | Against HIV | [29] | ||||
| Hydroclathrus clathratus | Sulphated polysaccharides | Against HSV | [27] | ||||
| Leathesia difformis | Sulphated polysaccharides | Against Influenza |
[30] | ||||
| Lobophora variegate | Sulphated fucans | Against HIV | [29] | ||||
| Padina tetrastromatica | Sulphated polysaccharides | Against HSV | [31] | ||||
| [ | Sphacelaria indica | Sulphated polysaccharides | Against HSV | [32] | |||
| Spachnidium rugosum | Sulphated polysaccharides | Against HSV | [33] | ||||
| Spatoglossum schroederi | Sulphated polysaccharides | Against HIV | [29] | ||||
| Stoechodperumum magiatum | Sulphated polysaccharides | Against HSV | [34] | ||||
| Undaria pinnatifida | Sulphated polysaccharides | Against HSV | [29,35][29][35] | ||||
| Sargassum patens | Sulphated polysaccharides | Against HSV | [17] | ||||
| Undaria pinnatifida | Sulphated polysaccharides | Against HSV | [12] | ||||
| Callophyllis variegate | Sulphated galactans | Against HSV | [36] | ||||
| Undaria pinnatifida | Sulphated polysaccharides | Against HIV | [33] | ||||
| Adenocystis utricularis | Fucoidans | Against HSV | [37] |
Table 2 provides a comprehensive review of the literature for antiviral activity of different Rhodophyceae along with their active metabolite. The review indicates that species belonging to Rhodophyceae is potent against HIV and both types of HSV viruses.
Table 2. A review of antiviral activity of macroalgae—Rhodophyceae.
A review of antiviral activity of macroalgae—Rhodophyceae.
| 51 | ||||
| ] | ||||
| Caulerpa brachypus | ||||
| Sulphated Polysaccharides | Against HSV-1 | [ | 52] | |
| Caulerpa scapelliformis | Sulphated Polysaccharides | Against HSV-1 | ||
| Caulerpa okamurai | Sulphated Polysaccharides | Against HSV-1 | ||
| Chaetomorpha crassa | Sulphated Polysaccharides | Against HSV-1 | ||
| Chaetomorpha spiralis | Sulphated Polysaccharides | Against HSV-1 | ||
| Monostroma nitidum, | Sulphated Polysaccharides | Against HSV-1 | ||
| Codium adhaerens | Sulphated Polysaccharides | Against HSV-1 | ||
| Codium latum | Sulphated Polysaccharides | Against HSV-1 | ||
| Macroalgae Taxa |
Macroalgae Species | Bioactive Metabolites | Antiviral Activity | Reference |
|---|---|---|---|---|
| Rhodophyceae | Gigartina atropupurea | Sulphated Polysaccharides | Against HSV | [33] |
| Chondria sulphated polysaccharides | Peptides (Condriamide A) | Against HSV | [38] | |
| Schizymenia binderi | Sulphated Galactan | Against HSV | [39] | |
| Plocamium cartilagineum | Sulphated Polysaccharides | Against HSV | [33] | |
| Gracilaria corticate | Sulphated Polysaccharides (Galactan Sulphates) |
Against HSV | [40] | |
| Sebdeniia polydactyla | Sulphated Polysaccharides | Against Influenza, Herpes, HIV |
[31] | |
| Nemalion helminthoides | Sulphated Polysaccharides | Against Influenza, Herpes, HIV |
[41] | |
| Sphaerococcus coronopifolius | Sulphated Polysaccharides | Against Influenza, Herpes, HIV |
[42] | |
| Boergeseniella thuyoides | Sulphated Polysaccharides | Against Influenza, Herpes, HIV |
[42] | |
| _ | Sulfated Xylomannan | Against HSV-1 & HSV-2 |
[43] | |
| Bryopsis sulphated polysaccharides |
Cyclic Depsipeptide (Kahalalide F) | Against HIV | [44] | |
| Cryptonemia crenulate | Sulphated Polysaccharides | Against HSV-1 | [45] | |
| Gelidium cartilagenium | Sulphated Polysaccharides | Against Influenza. | [46] | |
| Grateloupia filicina | Sulfated GA lactones | Against HIV | [27] | |
| Stenogramme interrupta | Carrageenans | Against HSV-1 & HSV-2 |
[11] | |
| Asparagopsis armata | Sulfated agaran | Against HSV-1 | [47] | |
| Bostrychia montagnei | Sulfated agarans | Against HSV-1 & HSV-2 |
[48] | |
| Gymnogongrus torulosus | DL- hybrid galactans | Against HSV-2, dengue virus 2 |
[14] | |
| Gracilaria corticata | Sulfated agarans | Against HSV-1 & HSV-2 | [40] | |
| Grateloupia longifolia | Sulfated Galactones | Against HIV | [27] | |
| Sphaerococcus coronopifolius | Sulphated Polysaccharides | Against HIV & HSV-1 | [42] | |
| Boergeseniella boergesen | Sulphated Polysaccharides | Against HIV & HSV-1 | [42] | |
| Schizymenia binderi | Sulfated Galactan |
A comparison of the antiviral activity of the three taxa is shown in Figure 1 to illustrate the potential usage of different macroalgae for pharmaceutical purposes. Figure 1a shows that more than 50% of the review papers indicates the potency of Phaeophyceae against HSV. Whereas most of the Chlorophyceae species were reported to have antiviral activity against HSV-1 and HSV-2 as shown in Figure 1b. The antiviral activities of different Rhodophceae species are relatively equally distributed against HSV, HIV, HSV-1, HSV-2, and Influenza virus as shown in Figure 1c.
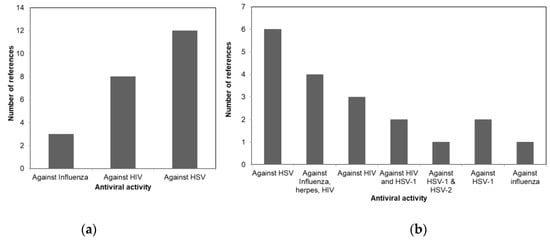
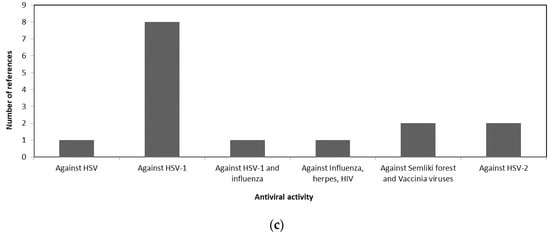


Figure 1. Antiviral activity of macroalgae (a) Phaeophyceae, (b) Chlorophyceae, and (c) Rhodophyceae.
The mechanism of action of sulphated polysaccharides against viral infection is explained as one of three ways; the first is by obstructing the virus from entering the cell. The second is by exhibiting virucidal activity. The third is by slowing down the syncytia formation. The multi-nucleate enlarged cell formed by syncytia is a result from fusion of a virally infected cell with neighboring host cells [53].
A detailed explanation of the mechanism of action of sulphated polysaccharide as antivirals has been explained by Wang et al. [54] who identified five mechanisms of action against a virus. These mechanisms were (a) direct viricidal action through the formation of an irreversible viral–polysaccharide complex, (b) inhibition of the viral adsorption by the host cell, (c) inhibition of virus uncoating, (d) hindering virus transcription inside the host cell, and (e) improvement of the host antiviral immune response by stimulation of antiviral immune factors.
Recently, Hans et al. [55] elaborated on the antiviral mechanism of marine sulphated polysaccharides. They explained four different ways in which a virus infection to the host cell can be inhibited by a sulphated polysaccharide. The first mechanism is the inhibition of attachment of the virus surface to the host cell through interaction of the negatively charged sulphated polysaccharide with the positively charged virus surface instead of its interaction with the negatively charged host cell. The second mechanism involves the inhibition of viral penetration into the host cell through the interaction between the sulphated marine polysaccharides and the virus receptors. The third mechanism was explained by the inhibition of virus uncoating inside the host cell through binding to the viral capsid that is formed inside the host cell. The final mechanism involves inhibition of the viral transcription in the host cell in case it managed to become uncoated through the interference with the replication enzymes such as reverse transcriptase enzyme.
The potency of the antiviral activity of macroalgae is determined by several structural factors of the sulphated polysaccharide, first, the carbohydrate backbone: molecular weight, linearity, the flexibility of the carbohydrate chain, and the influence of hydrophobic sites. Second, the structure of the anionic groups: carboxyl or sulphate groups, degree of sulphation, and the distribution of sulphate groups in the carbohydrate backbone [56].
The same was confirmed by Adhikari et al. [34]. They claim that sulphated polysaccharide’s antiviral activity depends on its molecular weight, constituent sugar, and the sulphation degree where low or absent sulphation indicates weak or non-antiviral activity.
Antioxidant Activity of Macroalgae
The oxidation process is a chemical reaction that involves the transfer of hydrogen atoms or oxygen atoms or electrons. This oxidation process might damage lipid membrane, protein, and deoxyribonucleic acid molecules, causing tissue injury in organisms. The term antioxidant refers to any compound that stops the oxidation process by hindering the reaction of a substance with dioxygen or any compound that inhibits the free radical reaction [57].
Pharmaceutically, antioxidants were used to block oxidation reaction initiation using high-energy molecules [58]. Since most of the organisms have antioxidant activity to defend themselves against oxidative damages, the bioactive compounds that marine organisms produce could play an essential role in the pharmaceutical industry.
Kohen and Nyska [59] claim that the sulphated polysaccharides in the cell wall of macroalgae do not occur in land plants, and their antioxidant properties may play an essential role against various diseases such as aging processes, chronic inflammation, and cardiovascular disorders.
Macroalgae are rich in sulphated polysaccharides such as fucoidan in brown algae, ulvan in green algae, and carrageenan in red algae. The sulphated polysaccharides in the cell wall of macroalgae have antioxidant activities, and therefore pharmaceutical antioxidants can be derived from macroalgae [60,61][60][61].
The antioxidant capacity of sulphated polysaccharide derived from marine red algae Porphyra haitanensis has been observed in aging mice [62]. It has also been reported that some natural antioxidants precede synthetic ones in potency; for example, Kim et al. [63] concluded that the sulphated polysaccharides of Sargassum fulvellum (Phaeophyceae), is a more potent nitric oxide scavenger than commercial antioxidants such as butylated hydroxyanisole.
Additionally, De Souza et al. [64] observed sulphated polysaccharides antioxidant capacity, where fucoidan and Fucans polysaccharides from Fucus vesiculosus and Padina gymnospora, respectively, had inhibitory effects on hydroxy radical and superoxide radical formation. The same was emphasized by Rocha de Souza et al. [65], who demonstrated a positive correlation between sulphated polysaccharide content and the antioxidant activity of macroalgae.
A positive correlation has been reported for sulphate content and superoxide radical scavenging activity in fucoidan fractions obtained from a brown alga Laminaria japonica [66]. Therefore, the pharmaceutical industry had shown a great interest in developing antioxidants from natural sources to waive the health hazards associated with synthetic antioxidants
Carrageenans antioxidant activity extracted from macroalgae has been studied with Alpha Carrageenan exhibiting antioxidant and free radical scavenging activity [67]. Macroalgae exhibit antioxidant properties that play an essential role in fighting cancer, chronic inflammation, and several other diseases. This finding provides a basis for further experiments on identifying sulphated polysaccharides with relatively high antioxidant activities [67].
The antioxidant potency of a sulphated polysaccharide was related to its chemical structure. For example, Zhang et al. [62] argue that sulphated polysaccharides antioxidant activity depends on their structural features such as the degree of sulphation, molecular weight, type of the major sugar, and glycosidic branching.
Qi et al. [68] have prepared different molecular weight ulvan from Ulva pertusua (Chlorophyceae) by hydrogen peroxide degradation and their antioxidant activities were investigated. Their results showed that low molecular weight ulvan have potent antioxidant activity. This is because low molecular weight sulphated polysaccharides may incorporate into the cells more efficiently and donate protons effectively compared to high molecular weight sulphated polysaccharides. Similarly, Sun et al. [69] and Chattopadhyay et al. [70] confirmed experimentally that low molecular weight sulphated polysaccharides have shown potent antioxidant activity compared to high molecular-weight sulphated polysaccharides.
In addition to the polysaccharides, authors claim that polyphenols, bromophenols, and mycosporine-like amino acids extracted from macroalgae also exhibit antioxidant properties [71,72][71][72]. Polyphenols are classified into distinct groups based on their structure, such as the flavonoids, phenolic acids, stilbenes, and lignans [73]. For example, Zubia et al. [74] demonstrated the antioxidant properties of Lobophora variegata due to its bromophenols and phenols content. Similarly, in brown algae, phlorotannins, a group of polyphenols that consists of polymers of phloroglucinol was reported to have radical scavenging capabilities [75].
The antioxidant activity of polyphenols in macroalgae was further confirmed by Tierney et al. [4]. Macroalgae exhibit antioxidant properties due to their possession of polyphenols, alkaloids, halogenated compounds. However, researchers also argue that alkaloids and halogenated compounds are more potent antimicrobial agents than antioxidants [76]. A synergy in antioxidant activity can only occur due to the coexistence of alkaloids and polyphenols in a macroalgae bioactive extract [77]. The same was confirmed by Abdel-Karim et al. [78] who concluded that the antioxidant capacity of bioactive compounds such as alkaloids and polyphenols extracted from macroalgae was mainly correlated to their phenolic content.
Table 4 provides a comprehensive review of the literature for antioxidant activity of different Phaeophyceae, Rhodophyceae, and Chlorophyceae species along with the active metabolite corresponding to the antioxidant activity.
Table 4. A review of antioxidant properties of different macroalgae species.
References
- Chan, C.-X.; Ho, C.-L.; Phang, S.-M. Trends in seaweed research. Trends Plant Sci. 2006, 11, 165–166.
- Jha, R.K.; Zi-Rong, X. Biomedical Compounds from Marine organisms. Mar. Drugs 2004, 2, 123–146.
- Chew, Y.L.; Lim, Y.Y.; Omar, M.; Khoo, K. Antioxidant activity of three edible seaweeds from two areas in South East Asia. LWT 2008, 41, 1067–1072.
- Tierney, M.S.; Croft, A.K.; Hayes, M. A review of antihypertensive and antioxidant activities in macroalgae. Bot. Mar. 2010, 53.
- Balina, K.; Romagnoli, F.; Blumberga, D. Seaweed biorefinery concept for sustainable use of marine resources. Energy Procedia 2017, 128, 504–511.
- Barzkar, N.; Jahromi, S.T.; PoorSaheli, H.B.; Vianello, F. Metabolites from Marine Microorganisms, Micro, and Macroalgae: Immense Scope for Pharmacology. Mar. Drugs 2019, 17, 464.
- Gerber, P.; Dutcher, J.D.; Adams, E.V.; Sherman, J.H. Protective Effect of Seaweed Extracts for Chicken Embryos Infected with Influenza B or Mumps Virus. Exp. Biol. Med. 1958, 99, 590–593.
- Witvrouw, M.; Desmyter, J.; De Cleroq, E. Antiviral portraitseries: 4. Polysulfates as inhibitors of HIV and other envelopedviruses. Antivir. Chem. Chemother. 1994, 94, 345–359.
- Witvrouw, M.; De Clercq, E. Sulfated Polysaccharides Extracted from Sea Algae as Potential Antiviral Drugs. Gen. Pharmacol. Vasc. Syst. 1997, 29, 497–511.
- Carlucci, M.; Scolaro, L.; Damonte, E. Inhibitory Action of Natural Carrageenans on Herpes simplex Virus Infection of Mouse Astrocytes. Chemotherapy 1999, 45, 429–436.
- Cáceres, P.J.; Carlucci, M.J.; Damonte, E.B.; Matsuhiro, B.; Zuniga, E.A. Carrageenans from chileansamples of Stenogramme interrupta (Phyllophoraceae): Structural analysis and biological activity. Phytochemistry 2000, 53, 81–86.
- Thompson, K.D.; Dragar, C. Antiviral activity of Undaria pinnatifida against herpes simplex virus. Phytotherapy Res. 2004, 18, 551–555.
- Ponce, N.M.; Pujol, C.A.; Damonte, E.B.; Flores, M.L.; Stortz, C.A. Fucoidans from the brown seaweed Adenocystis utricularis: Extraction methods, antiviral activity and structural studies. Carbohydr. Res. 2003, 338, 153–165.
- Pujol, C.; Estevez, J.M.; Carlucci, M.J.; Ciancia, M.; Cerezo, A.S.; Damonte, E.B. Novel DL-Galactan Hybrids from the Red Seaweed Gymnogongrus Torulosusare Potent Inhibitors of Herpes Simplex Virus and Dengue Virus. Antivir. Chem. Chemother. 2002, 13, 83–89.
- Ono, L.; Wollinger, W.; Rocco, I.M.; Coimbra, T.L.; Gorin, P.A.; Sierakowski, M.-R. In vitro and in vivo antiviral properties of sulfated galactomannans against yellow fever virus (BeH111 strain) and dengue 1 virus (Hawaii strain). Antivir. Res. 2003, 60, 201–208.
- Ohta, Y.; Lee, J.-B.; Hayashi, K.; Hayashi, T. Isolation of Sulfated Galactan from Codium fragile and Its Antiviral Effect. Biol. Pharm. Bull. 2009, 32, 892–898.
- Zhu, W.; Chiu, L.; Ooi, V.; Chan, P.; Ang, P. Antiviral property and mechanisms of a sulphated polysaccharide from the brown alga Sargassum patens against Herpes simplex virus type 1. Phytomedicine 2006, 13, 695–701.
- Vo, T.-S.; Kim, S.-K. Potential Anti-HIV Agents from Marine Resources: An Overview. Mar. Drugs 2010, 8, 2871–2892.
- Jiao, G.; Yu, G.; Zhang, J.; Ewart, H.S. Chemical Structures and Bioactivities of Sulfated Polysaccharides from Marine Algae. Mar. Drugs 2011, 9, 196–223.
- Pati, M.P.; Das Sharma, S.; Nayak, L.; Panda, C.R. Uses of seaweed and its application to human welfare: A review. Int. J. Pharm. Pharm. Sci. 2016, 8, 12.
- Grassauer, A.; Prieschl-Grassauer, E.; Biotech, A.G. Antiviral Composition Comprising a Sulfated Polysaccharide. U.S. Patent No. 10,342,820, 5 March 2009.
- Zaporozhets, T.S.; Besednova, N.N. Biologically active compounds from marine organisms in the strategies for combating coronaviruses. AIMS Microbiol. 2020, 6, 470–494.
- Ahn, G.; Kim, K.N.; Cha, S.H.; Song, C.B.; Lee, J.; Heo, M.S.; Yeo, I.K.; Lee, N.H.; Jee, Y.H.; Kim, J.S.; et al. Antioxidant activities of phlorotannins purified from Ecklonia cava on free radical scavenging using ESR and H2O2-mediated DNA damage. Eur. Food Res. Technol. 2007, 226, 71–79.
- Artan, M.; Li, Y.; Karadeniz, F.; Lee, S.H.; Kim, M.M.; Kim, S.K. Anti-HIV-1 activity of phloroglucinol derivative, 6,6′-bieckol, from Ecklonia cava. Bioorganic Med. Chem. 2008, 16, 7921–7926.
- Barbosa, J.P.; Pereira, R.C.; Abrantes, J.L.; Cirne dos Santos, C.C.; Rebello, M.A.; Frugulhetti, I.C.; Texeira, V.L. In vitro antiviral diterpenes from the Brazilian brown alga Dictyota pfaffi. Plant Med. 2004, 70, 856–860.
- Ryu, Y.B.; Jeong, H.J.; Yoon, S.Y.; Park, J.-Y.; Kim, Y.M.; Park, S.-J.; Rho, M.-C.; Kim, S.-J.; Lee, W.S. Influenza Virus Neuraminidase Inhibitory Activity of Phlorotannins from the Edible Brown Alga Ecklonia cava. J. Agric. Food Chem. 2011, 59, 6467–6473.
- Wang, S.; Bligh, S.; Shi, S.; Wang, Z.; Hu, Z.; Crowder, J.; Branford-White, C.; Vella, C. Structural features and anti-HIV-1 activity of novel polysaccharides from red algae Grateloupia longifolia and Grateloupia filicina. Int. J. Biol. Macromol. 2007, 41, 369–375.
- Mandal, P.; Mateu, C.G.; Chattopadhyay, K.; Pujol, C.A.; Damonte, E.B.; Ray, B. Structural features and antiviral activityof sulphated fucans from the brown seaweed Cystoseira indica. Antivir. Chem. Chemother. 2007, 18, 153–162.
- Queiroz, K.C.S.; Medeiros, V.P.; Queiroz, L.S.; Abreu, L.R.D.; Rocha, H.A.O.; Ferreira, C.V.; Juca, M.B.; Aoyama, H.; Leite, E.L. Inhibition of reverse transcriptase activity of HIV by polysaccharides of brown algae. Biomed. Pharmacother. 2008, 62, 303–307.
- Feldman, S.C.; Reynaldi, S.; Stortz, C.A.; Cerezo, A.S.; Damont, E.B. Antiviral properties of fucoidan fractions from Leathesia difformis. Phytomedicine 1999, 6, 335–340.
- Ghosh, T.; Chattopadhyay, K.; Marschall, M.; Karmakar, P.; Mandal, P.; Ray, B. Focus on antivirally active sulfated polysaccharides: From structure-activity analysis to clinical evaluation. Glycobiology 2009, 19, 2–15.
- Bandyopadhyay, S.S.; Navid, M.H.; Ghosh, T.; Schnitzler, P.; Ray, B. Structural features and in vitro antiviral activities of sulfated polysaccharides from Sphacelaria indica. Phytochemistry 2011, 72, 276–283.
- Harden, E.A.; Falshaw, R.; Carnachan, S.M.; Kern, E.R.; Prichard, M.N. Virucidal activity of polysaccharide extracts from four algal species against herpes simplex virus. Antivir. Res. 2009, 83, 282–289.
- Adhikari, U.; Mateu, C.G.; Chattopadhyay, K.; Pujol, C.A.; Damonte, E.B.; Ray, B. Structure and antiviral activity of sulfated fucans from Stoechospermum marginatum. Phytochemistry 2006, 67, 2474–2482.
- Cooper, R.; Dragar, C.; Elliot, K.; Fitton, J.H.; Godwin, J.; Thompson, K. GFS, a preparation of Tasmanian Undaria pinnatifida is associated with healing and inhibition of reactivation of Herpes. BMC Complementary Altern. Med. 2002, 2, 11.
- Rodríguez, M.C.; Merino, E.R.; Pujol, C.A.; Damonte, E.B.; Cerezo, A.S.; Matulewicz, M.C. Galactans from cystocarpic plants of the red seaweed Callophyllis variegata (Kallymeniaceae, Gigartinales). Carbohydr. Res. 2005, 340, 2742–2751.
- Ponce, N.M.A.; Stortz, C.A. A Comprehensive and Comparative Analysis of the Fucoidan Compositional Data across the Phaeophyceae. Front. Plant Sci. 2020, 11.
- Palermo, G.; Joris, H.; Devroey, P.; Van Steirteghem, A.C. Induction of acrosome reaction in human spermatozoa used for subzonal insemination. Hum. Reprod. 1992, 7, 248–254.
- Matsuhiro, B.; Conte, A.F.; Damonte, E.B.; Kolender, A.A.; Matulewicz, M.C.; Mejías, E.G.; Zúñiga, E.A. Structural analysis and antiviral activity of a sulfated galactan from the red seaweed Schizymenia binderi (Gigartinales, Rhodophyta). Carbohydr. Res. 2005, 340, 2392–2402.
- Mazumder, S.; Ghosal, P.K.; Pujol, C.A.; Carlucci, M.J.; Damonte, E.B.; Ray, B. Isolation, chemical investigation and antiviral activity of polysaccharides from Gracilaria corticata (Gracilariaceae, Rhodophyta). Int. J. Biol. Macromol. 2002, 31, 87–95.
- Pérez Recalde, M.; Noseda, M.D.; Pujol, C.A.; Carlucci, M.J.; Matulewicz, M.C. Sulfated mannans from the red seaweed Nemalion helminthoides of the South Atlantic. Phytochemistry 2009, 70, 1062–1068.
- Bouhlal, R.; Haslin, C.; Chermann, J.-C.; Colliec-Jouault, S.; Sinquin, C.; Simon, G.; Cerantola, S.; Riadi, H.; Bourgougnon, N. Antiviral Activities of Sulfated Polysaccharides Isolated from Sphaerococcus coronopifolius (Rhodophytha, Gigartinales) and Boergeseniella thuyoides (Rhodophyta, Ceramiales). Mar. Drugs 2011, 9, 1187–1209.
- Mandal, P.; Pujol, C.A.; Carlucci, M.J.; Chattopadhyay, K.; Damonte, E.B.; Ray, B. Anti-herpetic activity of a sulfated xylomannan from Scinaia hatei. Phytochemistry 2008, 69, 2193–2199.
- Haefner, B. Drugs from the deep: Marine natural products as drug candidates. Drug Discov. Today 2003, 8, 536–544.
- Talarico, L.B.; Zibetti, R.G.M.; Faria, P.C.S.; Scolaro, L.A.; Duarte, M.E.R.; Noseda, M.D.; Pujol, C.A.; Damonte, E.B. Anti-herpes simplex virus activity of sulfated galactans from the red seaweeds Gymnogongrus griffithsiae and Cryptonemia crenulata. Int. J. Biol. Macromol. 2004, 34, 63–71.
- Huheihel, M.; Ishanub, V.; Talb, J.; Arada, S.M. Activity of Porphyridium sp. polysaccharide against herpes simplex viruses in vitro and in vivo. J. Biochem. Biophys. Methods 2002, 50, 189–200.
- Haslin, C.; Lahaye, M.; Pellegrini, M.; Chermann, J.-C. In Vitro Anti-HIV Activity of Sulfated Cell-Wall Polysaccharides from Gametic, Carposporic and Tetrasporic Stages of the Mediterranean Red Alga Asparagopsis armata. Planta Med. 2001, 67, 301–305.
- Duarte, K.; Justino, C.; Gomes, A.; Rocha-Santos, T.; Duarte, A.C. Green Analytical Methodologies for Preparation of Extracts and Analysis of Bioactive Compounds. In Comprehensive Analytical Chemistry; Elsevier BV: Amsterdam, The Netherlands, 2014; Volume 65, pp. 59–78.
- Harnedy, P.A.; FitzGerald, R.J. Bioactive Proteins, Peptides, and Amino Acids from Macroalgae. J. Phycol. 2011, 47, 218–232.
- Ghosh, P.; Adhikaria, U.; Ghosala, P.K.; Pujolb, C.A.; Carluccib, M.J.; Damonteb, E.B.; Ray, B. In vitro anti-herpetic activity of sulfated polysaccharide fractions from Caulerpa racemosa. Phytochemistry 2004, 65, 3151–3157.
- Faulkner, D.J. Marine natural products. Nat. Prod. Rep. 2001, 18, 1–49.
- Lee, J.B.; Hayashi, K.; Hashimoto, M.; Nakano, T.; Hayashi, T. Novel antiviral fucoidan from sporophyll of Undaria pinnatifida (Mekabu). Chem. Pharm. Bull. 2004, 52, 1091–1094.
- Jane, P.; Bradford, M. Seaweed: Nature’s Secret for a Long and Healthy Life? Nutr. pract. 2006, 1–21.
- Wang, B.; Tong, G.Z.; Le Qu, Y.; Li, L. Microwave-Assisted Extraction and In Vitro Antioxidant Evaluation of Polysaccharides from Enteromorpha prolifera. Appl. Mech. Mater. 2011, 79, 204–209.
- Hans, N.; Malik, A.; Naik, S. Antiviral activity of sulfated polysaccharides from marine algae and its application in combating COVID-19: Mini review. Bioresour. Technol. Rep. 2021, 13, 100623.
- Damonte, E.B.; Matulewicz, M.C.; Cerezo, A.S. Sulfated Seaweed Polysaccharides as Antiviral Agents. Curr. Med. Chem. 2004, 11, 2399–2419.
- Ezeigbo, I.I.; Ezeja, M.; Madubuike, K.; Ifenkwe, D.; Ukweni, I.; Udeh, N.; Akomas, S. Antidiarrhoeal activity of leaf methanolic extract of Rauwolfia serpentina. Asian Pac. J. Trop. Biomed. 2012, 2, 430–432.
- Butterfield, D.; Castegna, A.; Pocernich, C.B.; Drake, J.; Scapagnini, G.; Calabrese, V. Nutritional approaches to combat oxidative stress in Alzheimer’s disease. J. Nutr. Biochem. 2002, 13, 444–461.
- Kohen, R.; Nyska, A. Invited Review: Oxidation of Biological Systems: Oxidative Stress Phenomena, Antioxidants, Redox Reactions, and Methods for Their Quantification. Toxicol. Pathol. 2002, 30, 620–650.
- Rupérez, P.; Ahrazem, O.; Leal, J.A. Potential Antioxidant Capacity of Sulfated Polysaccharides from the Edible Marine Brown SeaweedFucus vesiculosus. J. Agric. Food Chem. 2002, 50, 840–845.
- Wijesekara, I.; Pangestuti, R.; Kim, S.-K. Biological activities and potential health benefits of sulfated polysaccharides derived from marine algae. Carbohydr. Polym. 2011, 84, 14–21.
- Zhang, Q.-B.; Yu, P.-Z.; Zhou, G.-F.; Li, Z.-E.; Xu, Z.-H. Studies on antioxidant activities of fucoidan from Laminaria japonica. Chin. Trad. Herbal. Drugs 2003, 34, 824–826.
- Kim, S.H.; Choi, D.S.; Athukorala, Y.; Jeon, Y.J.; Senevirathne, M.; Rha, C.K. Antioxidant Activity of Sulphated Polysaccharides Isolated from Sargassum fulvellum. J. Food Sci. Nutr. 2007, 12, 65–73.
- De Souza, M.C.R. Antioxidant activity of fucanas and galactans extracted from seaweed. Master’s Thesis, Federal University of Rio Grande do Norte, Natal, Rio Grande do Norte, Brazil, 26 May 2008.
- Rocha de Souza, M.C.; Marques, C.T.; Dore, C.M.G.; da Silva, F.R.F.; Rocha, H.A.O.; Leite, E.L. Antioxidant activities of sulfated polysaccharides from brown and red seaweeds. J. Appl. Phycol. 2007, 19, 153–160.
- Wang, X.; Zhang, C.; Shi, F.; Hu, X. Purification and Characterization of Lipopolysaccharides. In Alzheimer’s Disease; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2010; Volume 53, pp. 27–51.
- Prajapati, V.D.; Maheriya, P.M.; Jani, G.K.; Solanki, H.K. RETRACTED: Carrageenan: A natural seaweed polysaccharide and its applications. Carbohydr. Polym. 2014, 105, 97–112.
- Qi, H.; Zhao, T.; Zhang, Q.; Li, Z.; Zhao, Z.; Xing, R. Antioxidant activity of different molecular weight sulfated polysaccharides from Ulva pertusa Kjellm (Chlorophyta). J. Appl. Phycol. 2005, 17, 527–534.
- Sun, L.; Wang, C.; Shi, Q.; Ma, C. Preparation of different molecular weight polysaccharides from Porphyridium cruentum and their antioxidant activities. Int. J. Biol. Macromol. 2009, 45, 42–47.
- Chattopadhyay, N.; Ghosh, T.; Sinha, S.; Chattopadhyay, K.; Karmakar, P.; Ray, B. Polysaccharides from Turbinaria conoides: Structural features and antioxidant capacity. Food Chem. 2010, 118, 823–829.
- Heo, S.-J.; Cha, S.-H.; Lee, K.-W.; Jeon, Y.-J. Antioxidant Activities of Red Algae from Jeju Island. ALGAE 2006, 21, 149–156.
- Nogueira, C.C.R.; Paixão, I.C.N.D.P.; Teixeira, V.L. Antioxidant Activity of Natural Products Isolated from Red Seaweeds. Nat. Prod. Commun. 2014, 9, 1031–1036.
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747.
- Zubia, M.; Robledo, D.; Freile-Pelegrin, Y. Antioxidant activities in tropical marine macroalgae from the Yucatan Peninsula, Mexico. J. Appl. Phycol. 2007, 19, 449–458.
- Kim, A.R.; Shin, T.S.; Park, J.Y.; Park, K.E.; Yoon, N.Y.; Kim, J.S.; Choi, J.S.; Jang, B.C.; Byun, D.S.; Park, N.K.; et al. Isolation and identification of phlorotannins from Ecklonia stolonifera with anti-oxidant and anti-inflammatory properties. J. Agric. Food Chem. 2009, 57, 3483–3489.
- Srivastava, N.; Saurav, K.; Mohanasrinivasan, V.; Kannabiran, K.; Singh, M. Antibacterial Potential of Macroalgae Collected from the Mandapam Coast. India Br. J. Pharmacol. Toxicol. 2010, 1, 72–76.
- Kosanić, M.; Ranković, B.; Stanojković, T. Biological activities of two macroalgae from Adriatic coast of Montenegro. Saudi J. Biol. Sci. 2015, 22, 390–397.
- Abdel-Karim, O.H.; Gheda, S.F.; Ismail, G.A.; Abo-Shady, A.M. Phytochemical Screening and antioxidant activity of Chlorella vulgaris. Delta J. Sci. 2020, 41, 81–91.
More
