Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Amina Yu and Version 4 by Amina Yu.
species belonging to the Phaeophyceae and Rhodophceae classes are primarily potent against herpes simplex virus, followed by human immunodeficiency virus and influenza virus.
- macro-algae
- antioxidant
- antiviral
- ulvan
- subcritical water extraction
1. Introduction
In 2004, the food and agriculture organization (FAO) introduced a taxa classification of macro-algae according to its pigmentation, brown (Phaeophyceae class), red (Rhodophyceae class), and green (Chlorophyceae class). Since then, different macro-algae classes have gained scholars’ attention for their ecological importance of supplying oxygen to the sea and usage in traditional medicine due to their perceived health benefits [1].
More recently, it has been claimed that macro-algae represent about 9% of biomedical compounds obtained from the sea [2]. Scholars explain that bioactive compounds of macro-algae such as polysaccharides have proven to have an effective antioxidant and antiviral activity. They argue that those polysaccharides have been developed as a chemical defense mechanism to the harsh environments in which they grow, such as variation in salinity, solar radiation and tidal waves, competition for space and nutrients [3][4][3,4].
Therefore, recent research argues that marine metabolites can shape the future of the bioeconomy [5] and might emerge as a new wave of promising drugs [6]. However, despite this claim, only a few studies have provided a systematic literature review on their antioxidant and antiviral activity along with their optimal extraction method. Therefore, this study fills a gap in the current literature by illustrating a comparative review of the antioxidant and antiviral activities of different macro-algae classes and identifying the active metabolite’s optimal extraction medium. Furthermore, the paper discusses the extraction methods of the active metabolites in macro-algae; specifically, the extraction of ulvan polysaccharide from the Ulva species to optimize their use in medicinal products.
2. Antiviral Activity of Macroalgae
Antiviral Activity of Macroalgae
The antiviral activity of macroalgae has been reported consistently early in the literature, for example, Gerber et al. [7] claimed the antiviral activity of macroalgae against influenza B and mumps virus. Similarly, Witvrouw et al. [8] reported that Aghardhiella tenera and Nothogenia fastigiata species of seaweed have antiviral activity towards human immunodeficiency virus (HIV), herpes simplex virus types 1 and 2 (HSV-1 and HSV-2), and respiratory syncytial virus (RSV). Moreover, Witvrouw and De Clercq [9] confirmed the inhibitory effect against the enveloped viral replication by the complex structures of sulphated polysaccharides in macroalgae. In the same line, authors report that carrageenan has a selective inhibitory effect against the enveloped virus and blocked the transmission of several viruses such as HIV, herpes simplex virus, human cytomegalovirus, and human rhinoviruses [10][11][10,11].
In the following decades, researchers confirmed the algal extract’s virucidal effect [12][13][14][12,13,14]. Scholars also confirmed that the low cytotoxicity, and successful use of antivirals from macroalgae in vaginal therapy had made its production for pharmaceutical use widely accepted. Similarly, Ono et al. [15] confirmed that sulphated polysaccharide extracted from macroalgae has anti-HIV activity and was able to inhibit flaviviruses such as dengue virus. Moreover, several researchers have confirmed the inhibitory effects of sulphated polysaccharides derived from macroalgae on the herpes simplex virus strains [16][17][16,17]. Additionally, Vo and Kim [18] as well as Jiao et al. [19], highlighted the association of sulphated polysaccharides from macroalgae with the antiviral activity. Similarly, Pati et al. [20] confirmed that sulphated polysaccharides such as carrageenan, fucoidans, and sulphated rhamno galactans successfully inhibited the enveloped viruses like HIV, and herbs.
Additionally, Grassauer and Prieschl-Grassauer [21] claimed that marine biomass such as carrageenan sulphated polysaccharide can facilitate the protection from the newly discovered coronavirus disease 2019 (COVID-19) which belongs to a family of enveloped viruses, or at least can be used as coating material for protective supplies such as masks and gloves. The same was confirmed by Zaporozhets et al. [22] who reported that the sulphated polysaccharides extracted from marine algae Saccharina japonica showed a significant antiviral activity against the coronavirus. Thus, a potential antiviral medicine can be developed from macroalgae biomass for augmenting the existing antivirals to combat emerging types and variants of enveloped viruses.
Table 1 provides a comprehensive review of the literature for antiviral activity of different Phaeophyceae along with their active metabolite. The review indicates that species belonging to Phaeophyceae are primarily potent against HSV, followed by HIV and influenza virus.
Table 1. A review of antiviral activity of macroalgae—Phaeophycea.
| Macroalgae Taxa |
Macroalgae Species | Bioactive Metabolites | Antiviral Activity |
Reference |
|---|
is potent against HIV and both types of HSV viruses.
Table 2. A review of antiviral activity of macroalgae—Rhodophyceae.
| Macroalgae Taxa |
Macroalgae Species | Bioactive Metabolites | Antiviral Activity | Reference |
|---|---|---|---|---|
| Phaeophyceae | Ecklonia cava | Phlorotannin (6,6′-Bieckol, 8,8′-bieckol) |
| [ |
| 39 |
| ] |
Table 3 provides a comprehensive review of the literature for antiviral activity of different Chlorophyceae along with their active metabolite. The data emphasize that species belonging to Chlorophyceae class are recorded by most of the scholars to have antiviral activity against HSV-1 and HSV-2.
Table 3. A review of antiviral activity of macroalgae—Chlorophyceae.
| Macroalgae Taxa |
Macroalgae Species | Bioactive Metabolites | Antiviral Activity | Reference | ||||
|---|---|---|---|---|---|---|---|---|
| Against HIV | [ | 23 | ] | [24][23,24] | ||||
| Chlorophyceae | Codium fragile | Polysaccharides | Against HSV-2 | |||||
| Dictyota caribaea horning & schnetter |
Sulphated Fucans | Against HIV | [25] | |||||
| Ecklonia cava | Phlorotannin (Phloroglucinol, eckol, 7-Phloroeckol, phlorofucofuroeckol, dieckol) |
Against | ||||||
| Rhodophyceae | Gigartina atropupurea | Sulphated Polysaccharides | Against HSV | [33] | ||||
| [ | 16 | ] | Chondria sulphated polysaccharides | Peptides (Condriamide A) | Against HSV | [38] | ||
| Ulva sulphated polysaccharides |
Peptides (Hexapeptide) | Against HSV | Influenza | [26] | ||||
| [ | 49 | ] | Schizymenia binderi | Sulphated Galactan | Against HSV | [ | ||
| Caulerpa racemose | Sulphated Polysaccharides | 39 | Against HSV-2] | [50] | Grateloupia filicina | Sulphated polysaccharides | Against HSV | [27] |
| Plocamium cartilagineum | Sulphated Polysaccharides | Against HSV | ||||||
| Ulva fasciata | Sulphated Polysaccharides | Against Semliki Forest & Vaccinia Viruses | [33 | [51] | Grateloupia longifolia | Sulphated polysaccharides | Against HIV | [27] |
| ] | ||||||||
| Gracilaria corticate | Sulphated Polysaccharides | |||||||
| Codium elongatum | (Galactan Sulphates) |
Sulphated PolysaccharidesAgainst HSV | Against Semliki Forest & Vaccinia Viruses[40] |
[51] | Adenocystis utricularis | Sulphated polysaccharides | Against HSV | [13] |
| Sebdeniia polydactyla | Sulphated Polysaccharides | Against Influenza, | ||||||
| Caulerpa brachypus | Sulphated Polysaccharides | Herpes, HIV | Against HSV-1[31] | [52] | Cystoseira indica | Sulphated polysaccharides | Against HSV | [ |
| Nemalion helminthoides | Sulphated Polysaccharides | |||||||
| Caulerpa scapelliformis | Against Influenza, | Herpes, HIV | 28] | |||||
| [ | 41 | ] | Sulphated Polysaccharides | Against HSV-1 | Dictyota mertensii | Sulphated polysaccharides | Against HIV | |
| Sphaerococcus coronopifolius | Sulphated Polysaccharides | Against Influenza, Herpes, HIV[29] |
||||||
| [ | 42 | ] | ||||||
| Caulerpa okamurai | Fucus vesiculosus | |||||||
| Sulphated Polysaccharides | Against HSV-1 | Boergeseniella thuyoidesSulphated polysaccharides | Against HIV | Sulphated Polysaccharides | Against Influenza, Herpes, HIV[29] |
|||
| [ | 42 | ] | ||||||
| Chaetomorpha crassa | Sulphated Polysaccharides | Against HSV-1 | Hydroclathrus clathratus | Sulphated polysaccharides | Against HSV | [27] | ||
| _ | Sulfated Xylomannan | Against HSV-1 & HSV-2 |
[43] | |||||
| Chaetomorpha spiralis | Sulphated Polysaccharides | Against HSV-1 | Leathesia difformis | Bryopsis sulphated Sulphated polysaccharides |
polysaccharides | Cyclic Depsipeptide (Kahalalide F)Against Influenza |
[30] | |
| Against HIV | [44] | |||||||
| Monostroma nitidum, | Sulphated Polysaccharides | Against HSV-1 | Lobophora variegate | Cryptonemia crenulateSulphated fucans | Against HIV | [29] | ||
| Sulphated Polysaccharides | Against HSV-1 | [45] | ||||||
| Codium adhaerens | Sulphated Polysaccharides | Against HSV-1 | Padina tetrastromatica | Sulphated polysaccharides | Against HSV | |||
| Gelidium cartilagenium | Sulphated Polysaccharides[31] | |||||||
| Against Influenza. | [46] | |||||||
| Codium latum | Sulphated Polysaccharides | Against HSV-1 | Sphacelaria indica | Sulphated polysaccharides | Against HSV | [32] | ||
| Grateloupia filicina | Sulfated GA lactones | Against HIV | [27] | Spachnidium rugosum | Sulphated polysaccharides | Against HSV | [33] | |
| Stenogramme interrupta | Carrageenans | Against HSV-1 & HSV-2 |
Spatoglossum schroederi | Sulphated polysaccharides | Against HIV | [29] | ||
| [ | 11 | ] | ||||||
| Asparagopsis armata | Sulfated agaran | Against HSV-1 | [47] | Stoechodperumum magiatum | Sulphated polysaccharides | Against HSV | [34] | |
| Bostrychia montagnei | Sulfated agarans | Against HSV-1 & HSV-2 |
[48] | Undaria pinnatifida | Sulphated polysaccharides | Against HSV | [29][35][29,35] | |
| Gymnogongrus torulosus | DL- hybrid galactans | Sargassum patens | Sulphated polysaccharides | Against HSV | [17] | |||
| Against HSV-2, | dengue virus 2 | [14] | ||||||
| Gracilaria corticata | Sulfated agarans | Against HSV-1 & HSV-2 | [40] | Undaria pinnatifida | Sulphated polysaccharides | Against HSV | [12] | |
| Grateloupia longifolia | Sulfated Galactones | Against HIV | [27] | Callophyllis variegate | Sulphated galactans | Against HSV | [36] | |
| Sphaerococcus coronopifolius | Sulphated Polysaccharides | Against HIV & HSV-1 | [42] | Undaria pinnatifida | Sulphated polysaccharides | Against HIV | [33] | |
| Boergeseniella boergesen | Sulphated Polysaccharides | Against HIV & HSV-1 | [42] | Adenocystis utricularis | Fucoidans | |||
| Schizymenia binderi | Against HSV | [37] |
Table 2 provides a comprehensive review of the literature for antiviral activity of different Rhodophyceae along with their active metabolite. The review indicates that species belonging to Rhodophyceae
| Sulfated Galactan |
| Against HSV |
| [ |
| 39 |
| ] |
A review of antiviral activity of macroalgae—Rhodophyceae.
| Macroalgae Taxa |
Macroalgae Species | Bioactive Metabolites | Antiviral Activity | Reference |
|---|---|---|---|---|
| Rhodophyceae | Gigartina atropupurea | Sulphated Polysaccharides | Against HSV | [33] |
| Chondria sulphated polysaccharides | Peptides (Condriamide A) | Against HSV | [38] | |
| Schizymenia binderi | Sulphated Galactan | Against HSV | [39] | |
| Plocamium cartilagineum | Sulphated Polysaccharides | Against HSV | [33] | |
| Gracilaria corticate | Sulphated Polysaccharides (Galactan Sulphates) |
Against HSV | [40 |
A comparison of the antiviral activity of the three taxa is shown in Figure 1 to illustrate the potential usage of different macroalgae for pharmaceutical purposes. Figure 1a shows that more than 50% of the review papers indicates the potency of Phaeophyceae against HSV. Whereas most of the Chlorophyceae species were reported to have antiviral activity against HSV-1 and HSV-2 as shown in Figure 1b. The antiviral activities of different Rhodophceae species are relatively equally distributed against HSV, HIV, HSV-1, HSV-2, and Influenza virus as shown in Figure 1c.
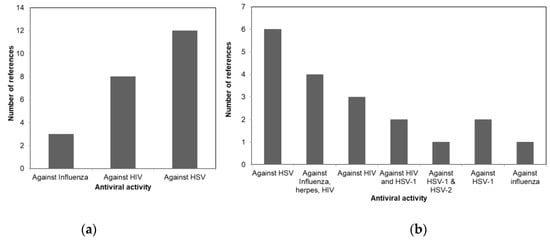
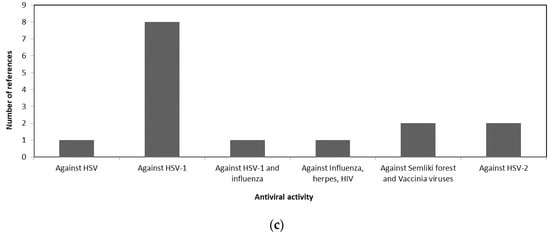


Figure 1. Antiviral activity of macroalgae (a) Phaeophyceae, (b) Chlorophyceae, and (c) Rhodophyceae.
The mechanism of action of sulphated polysaccharides against viral infection is explained as one of three ways; the first is by obstructing the virus from entering the cell. The second is by exhibiting virucidal activity. The third is by slowing down the syncytia formation. The multi-nucleate enlarged cell formed by syncytia is a result from fusion of a virally infected cell with neighboring host cells [53].
A detailed explanation of the mechanism of action of sulphated polysaccharide as antivirals has been explained by Wang et al. [54] who identified five mechanisms of action against a virus. These mechanisms were (a) direct viricidal action through the formation of an irreversible viral–polysaccharide complex, (b) inhibition of the viral adsorption by the host cell, (c) inhibition of virus uncoating, (d) hindering virus transcription inside the host cell, and (e) improvement of the host antiviral immune response by stimulation of antiviral immune factors.
Recently, Hans et al. [55] elaborated on the antiviral mechanism of marine sulphated polysaccharides. They explained four different ways in which a virus infection to the host cell can be inhibited by a sulphated polysaccharide. The first mechanism is the inhibition of attachment of the virus surface to the host cell through interaction of the negatively charged sulphated polysaccharide with the positively charged virus surface instead of its interaction with the negatively charged host cell. The second mechanism involves the inhibition of viral penetration into the host cell through the interaction between the sulphated marine polysaccharides and the virus receptors. The third mechanism was explained by the inhibition of virus uncoating inside the host cell through binding to the viral capsid that is formed inside the host cell. The final mechanism involves inhibition of the viral transcription in the host cell in case it managed to become uncoated through the interference with the replication enzymes such as reverse transcriptase enzyme.
The potency of the antiviral activity of macroalgae is determined by several structural factors of the sulphated polysaccharide, first, the carbohydrate backbone: molecular weight, linearity, the flexibility of the carbohydrate chain, and the influence of hydrophobic sites. Second, the structure of the anionic groups: carboxyl or sulphate groups, degree of sulphation, and the distribution of sulphate groups in the carbohydrate backbone [56].
The same was confirmed by Adhikari et al. [34]. They claim that sulphated polysaccharide’s antiviral activity depends on its molecular weight, constituent sugar, and the sulphation degree where low or absent sulphation indicates weak or non-antiviral activity.
3. Antioxidant Activity of Macroalgae
Antioxidant Activity of Macroalgae
The oxidation process is a chemical reaction that involves the transfer of hydrogen atoms or oxygen atoms or electrons. This oxidation process might damage lipid membrane, protein, and deoxyribonucleic acid molecules, causing tissue injury in organisms. The term antioxidant refers to any compound that stops the oxidation process by hindering the reaction of a substance with dioxygen or any compound that inhibits the free radical reaction [57].
Pharmaceutically, antioxidants were used to block oxidation reaction initiation using high-energy molecules [58]. Since most of the organisms have antioxidant activity to defend themselves against oxidative damages, the bioactive compounds that marine organisms produce could play an essential role in the pharmaceutical industry.
Kohen and Nyska [59] claim that the sulphated polysaccharides in the cell wall of macroalgae do not occur in land plants, and their antioxidant properties may play an essential role against various diseases such as aging processes, chronic inflammation, and cardiovascular disorders.
Macroalgae are rich in sulphated polysaccharides such as fucoidan in brown algae, ulvan in green algae, and carrageenan in red algae. The sulphated polysaccharides in the cell wall of macroalgae have antioxidant activities, and therefore pharmaceutical antioxidants can be derived from macroalgae [60][61][60,61].
The antioxidant capacity of sulphated polysaccharide derived from marine red algae Porphyra haitanensis has been observed in aging mice [62]. It has also been reported that some natural antioxidants precede synthetic ones in potency; for example, Kim et al. [63] concluded that the sulphated polysaccharides of Sargassum fulvellum (Phaeophyceae), is a more potent nitric oxide scavenger than commercial antioxidants such as butylated hydroxyanisole.
Additionally, De Souza et al. [64] observed sulphated polysaccharides antioxidant capacity, where fucoidan and Fucans polysaccharides from Fucus vesiculosus and Padina gymnospora, respectively, had inhibitory effects on hydroxy radical and superoxide radical formation. The same was emphasized by Rocha de Souza et al. [65], who demonstrated a positive correlation between sulphated polysaccharide content and the antioxidant activity of macroalgae.
A positive correlation has been reported for sulphate content and superoxide radical scavenging activity in fucoidan fractions obtained from a brown alga Laminaria japonica [66]. Therefore, the pharmaceutical industry had shown a great interest in developing antioxidants from natural sources to waive the health hazards associated with synthetic antioxidants
Carrageenans antioxidant activity extracted from macroalgae has been studied with Alpha Carrageenan exhibiting antioxidant and free radical scavenging activity [67]. Macroalgae exhibit antioxidant properties that play an essential role in fighting cancer, chronic inflammation, and several other diseases. This finding provides a basis for further experiments on identifying sulphated polysaccharides with relatively high antioxidant activities [67].
The antioxidant potency of a sulphated polysaccharide was related to its chemical structure. For example, Zhang et al. [62] argue that sulphated polysaccharides antioxidant activity depends on their structural features such as the degree of sulphation, molecular weight, type of the major sugar, and glycosidic branching.
Qi et al. [68] have prepared different molecular weight ulvan from Ulva pertusua (Chlorophyceae) by hydrogen peroxide degradation and their antioxidant activities were investigated. Their results showed that low molecular weight ulvan have potent antioxidant activity. This is because low molecular weight sulphated polysaccharides may incorporate into the cells more efficiently and donate protons effectively compared to high molecular weight sulphated polysaccharides. Similarly, Sun et al. [69] and Chattopadhyay et al. [70] confirmed experimentally that low molecular weight sulphated polysaccharides have shown potent antioxidant activity compared to high molecular-weight sulphated polysaccharides.
In addition to the polysaccharides, authors claim that polyphenols, bromophenols, and mycosporine-like amino acids extracted from macroalgae also exhibit antioxidant properties [71][72][71,72]. Polyphenols are classified into distinct groups based on their structure, such as the flavonoids, phenolic acids, stilbenes, and lignans [73]. For example, Zubia et al. [74] demonstrated the antioxidant properties of Lobophora variegata due to its bromophenols and phenols content. Similarly, in brown algae, phlorotannins, a group of polyphenols that consists of polymers of phloroglucinol was reported to have radical scavenging capabilities [75].
The antioxidant activity of polyphenols in macroalgae was further confirmed by Tierney et al. [4]. Macroalgae exhibit antioxidant properties due to their possession of polyphenols, alkaloids, halogenated compounds. However, researchers also argue that alkaloids and halogenated compounds are more potent antimicrobial agents than antioxidants [76]. A synergy in antioxidant activity can only occur due to the coexistence of alkaloids and polyphenols in a macroalgae bioactive extract [77]. The same was confirmed by Abdel-Karim et al. [78] who concluded that the antioxidant capacity of bioactive compounds such as alkaloids and polyphenols extracted from macroalgae was mainly correlated to their phenolic content.
Table 4 provides a comprehensive review of the literature for antioxidant activity of different Phaeophyceae, Rhodophyceae, and Chlorophyceae species along with the active metabolite corresponding to the antioxidant activity.
Table 4. A review of antioxidant properties of different macroalgae species.
A review of antioxidant properties of different macroalgae species.
| Macroalgae Taxa |
Macroalgae Species |
Bioactive Metabolites | Reference | ||||
|---|---|---|---|---|---|---|---|
| Phaeophyceae | Eisenia bicyclis | Polyphenols | [71,79] | ||||
| Rhodophyceae | Martensia fragilis | Alkaloids | [80] | ||||
| Phaeophyceae | Laminaria species | Phenolic compounds | [81] | ||||
| Phaeophyceae | Ecklonia cava | Phlorotannin (2,7-Phloroglucinol, 6,6′-bieckol) | [23,82] | ] | |||
| Phaeophyceae | E. kurome | Phlortotannin (dieckol) | [23] | Sebdeniia polydactyla | Sulphated Polysaccharides | Against Influenza, Herpes, HIV |
[ |
| Phaeophyceae | Padina perindusiata Thivy31] | ||||||
| Sulphated Fucans | [65] | Nemalion helminthoides | Sulphated Polysaccharides | ||||
| Phaeophyceae | Against Influenza, Herpes, HIV |
[ | Ecklonia stolonifera | Phlorotannin (Phlorofucofuroeckol A, 41] |
|||
| dieckol, dioxinodehydroeckol) | [ | 75] | Sphaerococcus coronopifolius | Sulphated Polysaccharides | |||
| Phaeophyceae | Against Influenza, Herpes, HIV |
[ | Ecklonia stolonifera | Phlorotannin (Phloroglucinol)42] | |||
| [ | 23 | ] | Boergeseniella thuyoides | Sulphated Polysaccharides | Against Influenza, Herpes, HIV |
[ | |
| Phaeophyceae | Lobophora | bromophenols and phenols42] | |||||
| [ | 74 | ] | _ | Sulfated Xylomannan | Against HSV-1 & | ||
| Phaeophyceae | Ecklonia stoloniferaHSV-2 | [ | Phlorotannin (2 Phloroeckol, eckol, phlorofucofuroeckol B, 6,6′-bieckol)43] |
||||
| [ | 83 | ] | Bryopsis sulphated polysaccharides |
Cyclic Depsipeptide (Kahalalide F) | |||
| Phaeophyceae | Fucus vesiculosusAgainst HIV | [ | Phlorotannin (Fucophlorethol A, tetrafucol A, trifucodiphlorethol A)44] |
||||
| [ | 84 | ] | Cryptonemia crenulate | Sulphated Polysaccharides | Against HSV-1 | [45] | |
| Phaeophyceae | Eisenia bicyclis | Phlorotannin (Triphlorethol A, 8,8′-Bieckol, phlorofucofuroeckol A, eckol, dieckol) |
[85] | Gelidium cartilagenium | Sulphated Polysaccharides | Against Influenza. | [46 |
| Phaeophyceae | Ishige okamurae] | ||||||
| Phlorotannin (Diphloroethohydroxycarmalol | [86] | Grateloupia filicina | Sulfated GA lactones | Against HIV | |||
| Phaeophyceae | Sargassum pallidum[27] | ||||||
| Sulphated Polysaccharides | [87] | Stenogramme interrupta | Carrageenans | Against HSV-1 & HSV-2 |
|||
| Phaeophyceae | Laminaria japonica | Sulphated Polysaccharides[11] | |||||
| [ | 62 | ,88] | Asparagopsis armata | Sulfated agaran | Against HSV-1 | [47] | |
| Phaeophyceae | Turbinaria ornata | Sulphated Polysaccharides | [89] | Bostrychia montagnei | |||
| RhodophyceaeSulfated agarans | Against HSV-1 & HSV-2 |
[ | Gigartina skottsbergi48] | ||||
| Sulphated Polysaccharides | [90] | Gymnogongrus torulosus | DL- hybrid galactans | Against HSV-2, dengue virus 2 |
[14 | ||
| Rhodophyceae | Gracilaria verrucose] | ||||||
| Sulphated Polysaccharides | [91] | Gracilaria corticata | Sulfated agarans | Against HSV-1 & HSV-2 | [40] | ||
| Rhodophyceae | Gracilaria opuntia | Azocinylmorpholinone | [92] | Grateloupia longifolia | Sulfated Galactones | Against HIV | [27] |
| Chlorophyceae | Ulva pertusa | Sulphated Polysaccharides (ulvans) | [93] | Sphaerococcus coronopifolius | Sulphated Polysaccharides | Against HIV & HSV-1 | [42] |
| Chlorophyceae | Ulva lactuca | Monounsaturated fatty acids (MUFA) derivatives | [ | Boergeseniella boergesen | Sulphated Polysaccharides | Against HIV & HSV-1 | [42] |
| 94 | ] | Schizymenia binderi | Sulfated Galactan | Against HSV |
