To improve the proficiency of energy systems in addition to increasing the usage of renewable energies, thermal energy storage (TES) is a strategic path.
- sorption
- heat storage
- thermo-chemical
- energy
- adsorbent materials
- salts
- composite
1. Overview
To improve the proficiency of energy systems in addition to increasing the usage of renewable energies, thermal energy storage (TES) is a strategic path. The present literature review reports an overview of the recent advancements in the utilization of salt hydrates (single or binary mixtures) and composites as sorbents for sorption heat storage. Starting by introducing various heat storage systems, the operating concept of the adsorption TES was clarified and contrasted to other technologies. Consequently, a deep examination and crucial problems related to the different types of salt hydrates and adsorbents were performed. Recent advances in the composite materials used in sorption heat storage were also reviewed and compared. A deep discussion related to safety, price, availability, and hydrothermal stability issues is reported. Salt hydrates display high theoretical energy densities, which are promising materials in TES. However, they show a number of drawbacks for use in the basic state including low temperature overhydration and deliquescence (e.g., MgCl2), high temperature degradation, sluggish kinetics leading to a low temperature rise (e.g., MgSO4), corrosiveness and toxicity (e.g., Na2S), and low mass transport due to the material macrostructure. The biggest advantage of adsorption materials is that they are more hydrothermally stable. However, since adsorption is the most common sorption phenomenon, such materials have a lower energy content. Furthermore, when compared to salt hydrates, they have higher prices per mass, which reduces their appeal even further when combined with lower energy densities. Economies of scale and the optimization of manufacturing processes may help cut costs. Among the zeolites, Zeolite 13X is among the most promising. Temperature lifts of 35–45 °C were reached in lab-scale reactors and micro-scale experiments under the device operating settings. Although the key disadvantage is an excessively high desorption temperature, which is problematic to attain using heat sources, for instance, solar thermal collectors. To increase the energy densities and enhance the stability of adsorbents, composite materials have been examined to ameliorate the stability and to achieve suitable energy densities. Based on the reviewed materials, MgSO4 has been identified as the most promising salt; it presents a higher energy density compared to other salts and can be impregnated in a porous matrix to prepare composites in order to overcome the drawbacks connected to its use as pure salt. However, due to pore volume reduction, potential deliquescence and salt leakage from the composite as well as degradation, issues with heat and mass transport can still exist. In addition, to increase the kinetics, stability, and energy density, the use of binary salt deposited in a porous matrix is suitable. Nevertheless, this solution should take into account the deliquescence, safety, and cost of the selected salts. Therefore, binary systems can be the solution to design innovative materials with predetermined sorption properties adapted to particular sorption heat storage cycles. Finally, working condition, desorption temperature, material costs, lifetime, and reparation, among others, are the essential point for commercial competitiveness. High material costs and desorption temperatures, combined with lower energy densities under normal device operating conditions, decrease their market attractiveness. As a result, the introduction of performance metrics within the scientific community and the use of economic features on a material scale are suggested.
2. Background
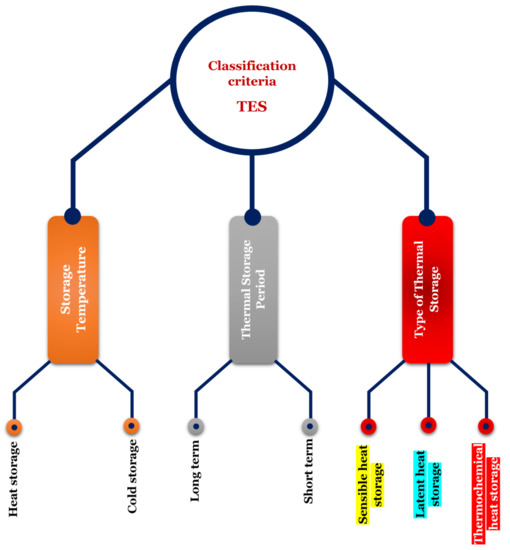
| SHS System | LHS System | TCES System | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Principle description | SHS storage involves increasing the temperature of an element and recovering this energy by dropping its temperature during the discharge phase. Then, we use the heat capacity, Cp, of the element, which is the amount of energy needed, per unit mass, to increase (or decrease) its temperature by 1 °C. The discharge temperature cannot be higher than the charge temperature. For applications where the operating temperature is between 0 and 100 °C, the most widely-used material is water, for example, in the domestic hot water tank of a home. Indeed, it is a non-toxic and inexpensive product. There is also the use of certain rocks or concrete. Beyond 100 °C, it is possible to use solid elements such as concrete at high temperatures or refractory ceramics, but the necessary volumes are important. Generally, we can find liquid storage systems for molten salts, pressurized water, or organic oils. For high temperature applications, molten salts are the most widely used material. This is due to their high volumetric heat capacity, a high boiling point, high temperature stability, and their vapor pressure being close to zero. Additionally, they are relatively cheap, readily available, neither toxic nor flammable, and can act as a heat transfer fluid as well as a TES material. However, they have certain disadvantages: they have a relatively high melting point (generally >200 °C), which results in them solidifying in pipes in the absence of a heat source and thus necessitates the installation of antifreeze systems; they also have high viscosity and low thermal conductivity compared to other fluids [27]. | SHS storage involves increasing the temperature of an element and recovering this energy by dropping its temperature during the discharge phase. Then, we use the heat capacity, Cp, of the element, which is the amount of energy needed, per unit mass, to increase (or decrease) its temperature by 1 °C. The discharge temperature cannot be higher than the charge temperature. For applications where the operating temperature is between 0 and 100 °C, the most widely-used material is water, for example, in the domestic hot water tank of a home. Indeed, it is a non-toxic and inexpensive product. There is also the use of certain rocks or concrete. Beyond 100 °C, it is possible to use solid elements such as concrete at high temperatures or refractory ceramics, but the necessary volumes are important. Generally, we can find liquid storage systems for molten salts, pressurized water, or organic oils. For high temperature applications, molten salts are the most widely used material. This is due to their high volumetric heat capacity, a high boiling point, high temperature stability, and their vapor pressure being close to zero. Additionally, they are relatively cheap, readily available, neither toxic nor flammable, and can act as a heat transfer fluid as well as a TES material. However, they have certain disadvantages: they have a relatively high melting point (generally >200 °C), which results in them solidifying in pipes in the absence of a heat source and thus necessitates the installation of antifreeze systems; they also have high viscosity and low thermal conductivity compared to other fluids [26]. | LHS is the amount of energy required to change the state of a solid, liquid or gas, called phase change material, PCM. In LHS systems, common transformations are from solid to liquid or from liquid to gas. The temperature range corresponding to the phase change of a PCM should be relatively small (20 to 80 °C). The materials used in LHS are numerous and allow working over a wide temperature range (example: 0 °C for water; 318 °C for sodium hydroxide). Latent heat storage in ‘low’ temperature range below 220 °C, ‘medium’ range up to 420 °C, and ‘high’ range greater than 420 °C is associated with a solar power tower as the point focus system. Materials for potential use as PCMs are mostly organic compounds, inorganic salts, and their eutectics. Inorganic materials such as salt hydrates, metals, and eutectics as well as organic compounds such as paraffin waxes, esters, acids, and alcohols have been studied [28]. According to the literature, molten salts have received more attention for heat storage applications than molten metals and alloys [29]. | LHS is the amount of energy required to change the state of a solid, liquid or gas, called phase change material, PCM. In LHS systems, common transformations are from solid to liquid or from liquid to gas. The temperature range corresponding to the phase change of a PCM should be relatively small (20 to 80 °C). The materials used in LHS are numerous and allow working over a wide temperature range (example: 0 °C for water; 318 °C for sodium hydroxide). Latent heat storage in ‘low’ temperature range below 220 °C, ‘medium’ range up to 420 °C, and ‘high’ range greater than 420 °C is associated with a solar power tower as the point focus system. Materials for potential use as PCMs are mostly organic compounds, inorganic salts, and their eutectics. Inorganic materials such as salt hydrates, metals, and eutectics as well as organic compounds such as paraffin waxes, esters, acids, and alcohols have been studied [27]. According to the literature, molten salts have received more attention for heat storage applications than molten metals and alloys [28]. |
TCES involves reversible reactions, endothermic in one direction and exothermic in the other direction. These may be physical or chemical phenomena, described as follows: A + ∆H ⇔ B + C During the storage process, the charging phase corresponds to an endothermic decomposition reaction of a chemical element A into two products B and C stored separately at room temperature. The phase of discharge corresponds to the exothermic synthesis of the chemical element A by association of the two components B and C. It can correspond to physisorption or chemisorption phenomena, but in all cases, the heat released comes from the breaking of the bonds between the various components. TCES can be applied to energy storage at less than 100 °C and between 100–400 °C, the physisorption being characterized by a low enthalpy (<50 kJ/mol of material) and chemisorption, by a high enthalpy (>100 kJ/mol of material). TCES is defined according to two criteria: is the process open or closed, and is the reactor integrated or separate from the storage system. In a closed circuit, the storage of the sorbate is an internal element of the process. In an open circuit, in contrast, there is a transfer of material with the outside of the process in order to supply the sorbate to the reactor. In both cases, sorbent storage is a component of the system. |
||||
| Volumetric energy density storage | Small (15–50 kWh/m | 3 | ) | Medium (50–100 kWh/m | 3 | ) | High (100–700 kWh/m | 3 | ) |
| Gravimetric energy density storage | Small (0.02–0.03 kWh/kg) | Medium (0.05–0.1 kWh/kg) | High (0.5–1.0 kWh/kg) | ||||||
| Capacity | 10–50 (kWh/t) | 50–100 (kWh/t) | 120–250 (kWh/t) | ||||||
| Power | 0.001–10.0 (MW) | 0.001–1.0 (MW) | 0.01–1.0 (MW) | ||||||
| Efficiency | 50–90% | 75–90% | 75–100% | ||||||
| Cost | 0.1–10 (€/kWh) | 10–50 (€/kWh) | 8–100 (€/kWh) | ||||||
| Storage temperature | Charging step temperature | Charging step temperature | Ambient temperature | ||||||
| Storage period | Limited due to thermal losses to surroundings | Limited due to thermal losses to surroundings | Theoretically unlimited | ||||||
| Energy transport | Shorter distance | Shorter distance | Theoretically long distance | ||||||
| Maturity | Industrial scale | Pilot-scale | Laboratory and pilot-scale | ||||||
| Technology | Simple | Simple | Complex |
3. Outlook
-
Decrease of unoccupied pores;
-
Probable deliquescence;
-
In terms of material, the strategic task is to decrease the prices of the available materials, with the aim to make TES sorption systems more competitive. At this stage, many efforts are devoted to the use of cheaper raw materials and diminishing the hydrophilicity of traditional zeolites, which needs high energy consumption (desorption temperature) that is unattainable, for instance, by traditional solar thermal collectors.
-
A deep examination on sorption heat storage is strongly required at diverse scales and several conditions can help to compare experimental studies in a standardized way. Recently, metal organic frameworks (MOFs) and aluminophosphates have shown a remarkable result that needs more focus, thanks to their promising features. Materials for TES sorption systems require more research to discover an appropriate active material with acceptable energy density, hydrothermal stability, and cyclability under the operating conditions of the device.
-
Leakage of salt from the composite; and
-
Degradation.
-
Composite materials are being studied in order to diminish the instabilities at salt hydrate material levels. If a high enough desorption temperature is achieved, the host matrices can be made of a porous material that can also act as an adsorbent. Small pore sizes, which are needed for the matrix to participate in the sorption process, result in little salt impregnated in the matrix. Ineffective materials including expanded graphite, sand, silica gel, and vermiculite, on the other hand, have been studied solely for structural support. Numerous studies have been published, but further work is being done to find promising working pairs. Diminished mass transport inside the matrix pores as well as salt deliquescence or overhydration can lead to active material leakage. Finally, the experimental conditions of the examined studies are heterogeneous and some of them are far from the characteristic conditions of low-temperature heat storage. Furthermore, more research is required to fully and deeply comprehend the kinetics and mechanisms that have occurred by using composite materials in TES sorption systems.
-
For upcoming studies on sorption heat storage systems, some critical points also should be considered: For instance, the energy density at different stages of the search need to be determined by setting a common reference temperature. In addition, along with the energy density, the required volume used must be defined.
-
Only a few studies have concentrated on the economic viability of the systems. This is partly due to the fact that analysis is always at the material and laboratory scale; thus, broad economic surveys will possibly lead to misleading results.
-
The economic feasibility of the system has not been well examined because there are many challenges not yet achieved at material and lab-scales. Consequently, broad economic surveys would likely lead to ambiguous outcomes. Nevertheless, the central indicators allied to material cost, system complexity, and auxiliary energy consumption system should be considered in order to have an idea about the estimation of system cost-effectiveness.
-
The cost of materials will already give an idea of how profitable and potential the system will be in a given application. For cost estimation, all components and auxiliary systems must be considered when increasing the scale. Supplementary economic considerations that can be highlighted at commercial scale (Prototype) linked to system operations such as lifetime and maintenance costs may perhaps also be involved to evaluate the rentability analysis.
References
- Leonzio, G. Solar systems integrated with absorption heat pumps and thermal energy storages: State of art. Renew. Sustain. Energy Rev. 2017, 70, 492–505.
- N’Tsoukpoe, K.E.; Liu, H.; Le Pierrès, N.; Luo, L. A review on long-term sorption solar energy storage. Renew. Sustain. Energy Rev. 2009, 13, 2385–2396.
- Matera, F.V.; Gatto, I.; Patti, A.; Passalacqua, E. Fuel cell performance assessment for closed-loop renewable energy systems. J. Energy Chem. 2016, 25, 531–538.
- Mustafa, A.; Lougou, B.G.; Shuai, Y.; Wang, Z.; Tan, H. Current technology development for CO2 utilization into solar fuels and chemicals: A review. J. Energy Chem. 2020, 49, 96–123.
- Nagel, T.; Beckert, S.; Lehmann, C.; Gläser, R.; Kolditz, O. Multi-physical continuum models of thermochemical heat storage and transformation in porous media and powder beds—A review. Appl. Energy 2016, 178, 323–345.
- Thomas, J.M.; Edwards, P.P.; Dobson, P.J.; Owen, G.P. Decarbonising energy: The developing international activity in hydrogen technologies and fuel cells. J. Energy Chem. 2020, 51, 405–415.
- Peng, X.; Root, T.W.; Maravelias, C.T. Storing solar energy with chemistry: The role of thermochemical storage in concentrating solar power. Green Chem. 2017, 19, 2427–2438.
- Aneke, M.; Wang, M. Energy storage technologies and real life applications—A state of the art review. Appl. Energy 2016, 179, 350–377.
- Desai, F.; Seyedhassantehrani, N.; Shagar, M.; Gu, S.; Asmatulu, R. Preparation and characterization of KOH-treated electrospun nanofiber mats as electrodes for iron-based redox-flow batteries. J. Energy Storage 2020, 27, 101053.
- Energy Information Administration (EIA). Energy Information Administration: Annual Energy Outlook 2018 with Projections to 2050; EIA: Washington, DC, USA, 2018.
- Pardo, P.; Deydier, A.; Anxionnaz-Minvielle, Z.; Rougé, S.; Cabassud, M.; Cognet, P. A review on high temperature thermochemical heat energy storage. Renew. Sustain. Energy Rev. 2014, 32, 591–610.
- Pereira da Cunha, J.; Eames, P. Thermal energy storage for low and medium temperature applications using phase change materials—A review. Appl. Energy 2016, 177, 227–238.
- Desai, F.; Sunku Prasad, J.; Muthukumar, P.; Rahman, M.M. Thermochemical energy storage system for cooling and process heating applications: A review. Energy Convers. Manag. 2021, 229, 113617.
- Berseneff, B.; Perrin, M.; Tran-Quoc, T.; Brault, P.; Mermilliod, N.; Hadjsaid, N.; Delaplagne, T.; Martin, N.; Crouzevialle, B. The significance of energy storage for renewable energy generation and the role of instrumentation and measurement. IEEE Instrum. Meas. Mag. 2014, 17, 34–40.
- Zhang, H.; Baeyens, J.; Cáceres, G.; Degrève, J.; Lv, Y. Thermal energy storage: Recent developments and practical aspects. Prog. Energy Combust. Sci. 2016, 53, 1–40.
- Abedin, A.H.; Rosen, M.A. Closed and open thermochemical energy storage: Energy- and exergy-based comparisons. Energy 2012, 41, 83–92.
- Zhang, Y.; Wang, R.; Li, T.; Zhao, Y. Thermochemical Characterizations of Novel Vermiculite-LiCl Composite Sorbents for Low-Temperature Heat Storage. Energies 2016, 9, 854.
- Solé, A.; Martorell, I.; Cabeza, L.F. State of the art on gas–solid thermochemical energy storage systems and reactors for building applications. Renew. Sustain. Energy Rev. 2015, 47, 386–398.
- Wongsuwan, W.; Kumar, S.; Neveu, P.; Meunier, F. A review of chemical heat pump technology and applications. Appl. Therm. Eng. 2001, 21, 1489–1519.
- Zhang, Y.N.; Wang, R.Z.; Zhao, Y.J.; Li, T.X.; Riffat, S.B.; Wajid, N.M. Development and thermochemical characterizations of vermiculite/SrBr2 composite sorbents for low-temperature heat storage. Energy 2016, 115, 120–128.
- Hasnain, S.M. Review on sustainable thermal energy storage technologies, Part I: Heat storage materials and techniques. Energy Convers. Manag. 1998, 39, 1127–1138.
- Hasnain, S.M. Review on sustainable thermal energy storage technologies, Part II: Cool thermal storage. Energy Convers. Manag. 1998, 39, 1139–1153.
- Dincer, I.; Rosen, M. Thermal Energy Storage Systems and Applications; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2011; ISBN 9780470747063.
- Shabgard, H.; Bergman, T.L.; Sharifi, N.; Faghri, A. High temperature latent heat thermal energy storage using heat pipes. Int. J. Heat Mass Transf. 2010, 53, 2979–2988.
- Ermis, K.; Erek, A.; Dincer, I. Heat transfer analysis of phase change process in a finned-tube thermal energy storage system using artificial neural network. Int. J. Heat Mass Transf. 2007, 50, 3163–3175.
- Caraballo, A.; Galán-Casado, S.; Caballero, Á.; Serena, S. Molten Salts for Sensible Thermal Energy Storage: A Review and an Energy Performance Analysis. Energies 2021, 14, 1197.
- Kenisarin, M.M. High-temperature phase change materials for thermal energy storage. Renew. Sustain. Energy Rev. 2010, 14, 955–970.
- Khare, S.; Dell’Amico, M.; Knight, C.; McGarry, S. Selection of materials for high temperature latent heat energy storage. Sol. Energy Mater. Sol. Cells 2012, 107, 20–27.
- Iten, M.; Liu, S. A work procedure of utilising PCMs as thermal storage systems based on air-TES systems. Energy Convers. Manag. 2014, 77, 608–627.
