1. Overview
Ovarian steroid-regulated cyclical regeneration of the endometrium is crucial for endometrial receptivity and embryo implantation, and it is dependent on the dynamic remodeling of the endometrial vasculature. Perivascular cells, including pericytes surrounding capillaries and microvessels and adventitial cells located in the outermost layer of large vessels, show properties of mesenchymal stem cells, and they are thus promising candidates for uterine regeneration. In this review, we discuss the structure and functions of the endometrial blood vasculature and their roles in endometrial regeneration, the main biomarkers and characteristics of perivascular cells in the endometrium, and stem cell-based angiogenetic therapy for Asherman’s syndrome.
2. Introduction
During a woman’s reproductive years, the endometrium dynamically undergoes around 400 cycles of morphological and functional changes, and the cyclic change of the endometrium is crucial for embryo implantation. The human endometrium comprises the upper two-thirds functional layer, which contains superficial epithelial cells and the underlying stroma, and the lower third basal layer. The functional layer uniquely goes through extensive proliferation and slough, while the basal layer does not shed during each menstrual cycle.
In the secretory phase, decidualization occurring in the endometrial stromal cells allows the maternal body to be immunotolerant to the allogenic embryo, and without pregnancy, the consequent menstruation enables the functional layer to be fully denuded and ready for regeneration, which only occurs in primates
[1]. Thereafter, the functional layer grows from the basal layer and is repaired in response to the rebound of estrogen in the proliferative phase
[2]. This process is regulated by ovarian steroids and accompanied by angiogenesis and vessel regression. Malformation of blood vessels is related to gynecological disorders, such as menoxenia and endometriosis, and can lead to failure in embryo implantation and infertility
[3][4]. Hence, the endometrium is a tissue where physiological injury and scar-free repair recurrently occur and for which the basal layer is postulated as a place possessing stem/progenitor cells
[5]. Once the uterus experiences severe trauma, such as curettage, cesarean section, hysteroscopic surgery, or infections that destroy the basal layer, fibroblasts are overactivated and secrete collagen, resulting in improper regeneration and formation of uterine scars and intrauterine adhesions (IUA)
[6][7][8][9].
Supplementation of stem/progenitor cells to compensate for the debilitating regeneration of the endometrium is considered to shed a sliver of light for IUA patients. Among several stem/progenitor cells under discussion, perivascular cells, including pericytes surrounding the capillaries and microvessels, as well as adventitial cells located in the outermost layer of large vessels, have been showing phenotypes of mesenchymal stem cells (MSCs) and regenerating capacities
[10].
This review introduces the development and structure of the endometrial vasculature, and examines how blood vessels vary in response to the cyclic change in ovarian steroids. The identity, characteristics, and different biomarkers of the perivascular cells and the associations between pericytes and adventitial cells are discussed. Furthermore, although with limited applications in both animal and clinical trials, the regenerating potential and underlying mechanisms of uterine repair based on perivascular cells in the treatment of severe Asherman’s syndrome (AS) or IUA are also discussed.
3. The Endometrial Vasculature
In the 10th week of pregnancy, bilateral Müllerian ducts fuse to form the uterus, and the endometrium completely differentiates from uterine mucosa by the 20th week
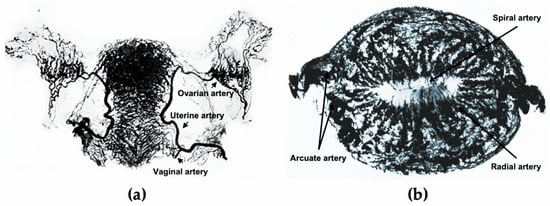
[11]. The development of the uterus is accompanied by the formation of the uterine vasculature, which comes from the uterine, ovarian, and vaginal arteries (a). The lateral branches emit anterior and posterior arcuate arteries, which then extend into the myometrium in the circumferential direction, nourishing two-thirds of the myometrium and divisions ending in capillary networks surrounding muscle fibers
[12]. After entering the endometrium, the number of spiral vessels sharply decreases compared with the myometrium, with dynamic variations in tortuosity during different menstrual phases (b)
[12].
Figure 1. The uterine vasculature. (a) Arterial blood supply of the ovaries, fallopian tubes, and uterus. (b) Radiographs of the transverse slice of the uterus in the mid-secretory phase, showing its arterial vasculature. a is edited from the figure by Norris, C.C., Clark, J.G., and Peters, D. Gonorrhea in Women. 1913. b is edited from the figure by Farrer-Brown, G., Beilby, J.O., and Tarbit, M.H. The Blood Supply of the Uterus. 1. Arterial Vasculature. J. Obstet. Gynaecol. Br. Commonw. 1970, 77, 673–681.
The formation of primitive blood vessels, also known as vasculogenesis, originates from endothelial progenitors called angioblasts, which are derived from mesodermal cells, differentiate into endothelial cells, and migrate and coalesce to form the early vascular plexus
[13]. The developing needs and microenvironment call for organ-specific endothelial specialization and mural cell coverage to deliver nutrients and gases, absorb and filter fluids, assist metabolic processes, and participate in the regulation of local immune cells
[14]. Thereafter, blood vessels undergo remodeling and expansion and form the vasculature network
[15]. The process of new blood vessels growing out from the existing vasculature is known as angiogenesis, which occurs in both physiological development process and pathological conditions
[16]. The vital role of angiogenesis has been widely studied during embryogenesis, where the establishment of circulation is no doubt a necessity for fetal development, and pathological processes such as inflammation and malignant tumor growth where the formation of new vessels both provides nourishing effects and supports immune responses
[17][18]. Despite considerable research exploring the processes of uterine development and fetal blood vessel formation, including both vasculogenesis and angiogenesis, the role of uterine-specific vasculature formation in uterine development remains unclear.
Variations in blood vessels rarely occur under physiological conditions in adults, except for the reproductive system. During the secretory phase of the menstrual cycle, the uterus is mainly supplied by the uterine artery, while a greater proportion of the uterus around the uterine horns is supplied by the ovarian artery during the proliferative phase
[19]. Periodic changes in the ratio of progesterone and estrogen from ovaries regulate the blood flow of uterus-supplying arteries, and the distribution of ovarian steroids delivered by peri-uterine vessels varies from that by systemic vessels, which shows the mutually reinforcing relationship between blood vessels and the reproductive apparatus
[19]. Furthermore, the morphology of the blood vessels dynamically changes with the menstrual cycle. Under physiological conditions, straight arterioles branch out of the arcuate arteries that are located in the uterine myometrium, which contain endothelial cells, surrounding smooth muscle cells (SMCs), and perivascular cells. The morphology of arterioles in the functional layer gradually varies from straight to spiral as the proliferative phase progresses, and the vessel wall becomes thinner as the arterioles pass through, leaving only vascular endothelial cells when these reach the subepithelial luminal surface
[20]. Endothelial cells that are not connected to SMCs or perivascular cells in the functionalis are susceptible to apoptosis when angiogenic factors are withdrawn, while the endothelial cells in the basalis and connected with pericytes remain stable
[20]. Factors including the VEGF family, hypoxia-inducible factor, angiopoietins, and fibroblast growth factor family are involved in the process of endometrial angiogenesis, regulating the proliferation and migration of endothelial cells
[20].
Three processes that angiogenesis prominently participates in are: (1) post-menstrual repair after vascular bed rupture, (2) endometrial growth during proliferative phase when the thickness of the endometrium increases by up to fourfold, and (3) the significant elongation and coiling of spiral arterioles during the secretory phase
[21]. Four forms of angiogenesis include sprouting, intussusception, vessel elongation, and circulating endothelial progenitor cells that incorporate into growing vessels
[22][23][24]. In spite of the initial and conventional efforts exerted into studies on vessel sprouting, it has been reported that non-sprouting angiogenesis plays a major role in the cyclic generation of the uterus. In particular, vessel elongation plays a major role in the mid–late proliferative phase, while the angiogenic pattern in the early–mid secretory phase tends to be intussusception
[25].
Angiogenesis is relatively active in the proliferative phase, and mitotic activity reflected by nuclear DNA synthesis is more intense in the functionalis than in the basalis layer throughout the proliferative phase
[26]. Interestingly, the rate of proliferation of endothelial cells does not significantly vary during the cycle and is still maintained in the late-secretory and menstrual phase, when there is no significant endometrial growth
[27][28], although the lack of a proliferation peak may probably be due to inter-individual variations
[26]. To determine the correlation between steroid changes and endometrial angiogenesis, a model was created by Nayak and Brenner, in which rhesus macaques were ovariectomized and supplemented with estradiol and progesterone to mimic the natural menstrual cycle
[29]. Proliferation of endothelial cells, as detected by Ki-67 and BrdU, increased 6-fold at 8–10 days after the withdrawal of progesterone, reached the bottom at menses, and remained steady in other stages. This result suggests that angiogenesis is an estrogen-dependent process that mainly occurs in the mid-proliferative phase and is in line with VEGF expression in the endometrial stroma at this phase
[29]. Given that the mechanism of angiogenesis in the endometrium is rather complex, measuring methods in addition to the proliferation of endothelial cells, such as laser Doppler imaging and visualization by pigment particle perfusion, should be introduced to study the growth pattern of blood vessels
[30].
In addition to angiogenesis, vessel maturation is another process that is required for regeneration after endometrial shedding. Mural cells, including vascular smooth muscle cells and pericytes, proliferate under the control of progesterone during the secretory phase, and wrap around endothelial cells, regulating angiogenesis and coordinating the function of blood vessels
[31]. In the late-secretory phase, perivascular cells form a thick layer and surround the spiral arterioles, not only resembling the decidual cells, but also maintaining the capacity to interact with endothelial cells
[32].
Aberrations in angiogenesis are associated with gynecological clinical consequences. The proliferation rate of endothelial cells instead of endometrial stromal cells, glandular cells, and surface epithelial cells has been reported to be higher in patients with menorrhagia
[33]. It has also been reported that intense vascularization exists in endometriosis implants, with a significantly higher secretion of VEGF in peritoneal fluids in the proliferative phase of the menstrual cycle
[34][35]. On the other hand, in severe IUA patients with a narrow uterine cavity, fibrotic scarring, vague uterine horns, and absence of glands, blood flow is poor in the pale endometrial tissues, where angiogenesis is completely blocked
[36]. Therefore, the dynamic balance between promotion and inhibition of blood vessel growth is inseparable from maintenance of the normal function of the endometrium.
4. Conclusions
In conclusion, the progenitor role of perivascular cells derived from human endometrium and a stem cell-based therapy for AS have been investigated in both murine model studies and clinical trials. Nevertheless, several crucial questions have yet to be solved. First, the relationship between human endometrial vasculature and uterine development under physiological conditions remains unknown. The synchronous development of uterus and blood vessels is to be further revealed by combining the means of embryo anatomy, multi-omics analysis, and single-cell sequencing. Second, the role of perivascular cells during the processes of uterine development and cyclic endometrial regeneration needs to be further explored by in vivo cell lineage tracing. Third, by combining with tissue engineering, perivascular cells are promising candidates for tissue repair under pathological conditions, while better seed cells and improved delivery methods need to be explored. Moreover, the mechanisms behind how the precursor cells mobilize the remaining local cells and how they induce microenvironmental changes to allow themselves to colonize remain unclear.