Microstructural phase evolution during melting and casting depends on the rate of cooling, the collective mobility of constituent elements, and binary constituent pairs. Parameters used in mechanical alloying and spark plasma sintering, the initial structure of binary alloy pairs, are some of the factors that influence phase evolution in powder-metallurgy-produced HEAs. Factors such as powder flowability, laser power, powder thickness and shape, scan spacing, and volumetric energy density (VED) all play important roles in determining the resulting microstructure in additive manufacturing technology. Large lattice distortion could hinder dislocation motion in HEAs, and this could influence the microstructure, especially at high temperatures, leading to improved mechanical properties in some HEAs. Mechanical properties of some HEAs can be influenced through solid solution hardening, precipitation hardening, grain boundary strengthening, and dislocation hardening. Despite the HEA system showing reliable potential engineering properties if commercialized, there is a need to examine the effects that processing routes have on the microstructure in relation to mechanical properties.
- high-entropy alloy
- powder metallurgy
- melting and casting
- microstructural evolution
- additive manufacturing
- solid solution strengthening
1. Introduction
The discovery and application of alloying and composite technology have made possible the achievement of various categories of materials that exhibit a wide range of properties. An example is a novel alloy system known as high-entropy alloys (HEAs). [1] defined HEAs, by composition, as alloys having at least five principal elements, wherein each has a concentration between 5 and 35 at.%. [2] also categorized HEAs based on elemental composition and configurational entropy.
Some categories of the HEAs studied are lanthanide HEAs [3][4][3,4], refractory HEAs (RHEAs) [5][6], and lightweight HEAs (LWHEAs) [6][7]. RHEAs are primarily developed for exceptionally high-temperature applications (up to 1400 °C), but with a disadvantage of high density. PGM-HEAs consist of precious elements (Au, Ag, Pt, Ir, Os, and Re), while LWHEAs are composed of low-density elements such as Li, Mg, Be, and Al.
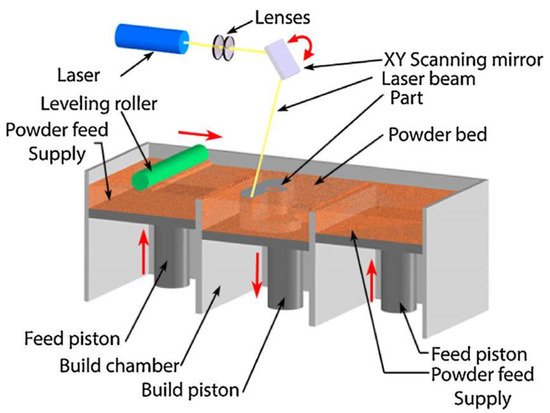
Over the past decade, material scientists have used several techniques in synthesizing HEAs, such as the melting and casting route, the powder metallurgy (PM) route, and additive manufacturing (AM) processing techniques. The PM process involving mechanical alloying (MA) and consolidation by spark plasma sintering (SPS) is usually used in attempts of achieving homogeneous microstructures in HEAs. In contrast, the AM fabrication route in recent years has received more attention in circumventing the flaws of other synthesis processes. The AM process is a flexible manufacturing technique with the capability of producing parts with complex geometries, finer microstructures, mass customization, and efficient material usage [7][11].
They are the high-entropy effect, sluggish diffusion effect, lattice distortion effect, and cocktail effect. Moreover, a fine precipitate and a controlled grain structure are usually formed as a result of the sluggish diffusion effect. The effect suggests that the pair distribution function directly relates to the distribution of the interatomic spacing on a local atomic level [8][15]. The properties of HEAs are known to be a result of the overall contributions of the constituent phases influenced by phase shape, phase distribution, and boundaries, as well as the properties of each phase [9][20].
There is no doubt that the basis of HEA design revolves around these so-called core effects. Hence, most HEAs studied have been derived from these basic principles [10][11][12][21,22,23]. Nevertheless, the validity of these core effects has been doubted by some researchers recently.
This makes the prediction of processing–structure relationships quite a challenge. The design approach adopted by most researchers does not follow a specific logic; rather, a number of these alloys are a result of a trial-and-error approach. Although attempts have been made to categorize these alloys according to the intended application, there still exists a multitude of alloys exhibiting a wide range of properties. This paper will also try to establish a structure–property relationship and link it to the processing route used.
2. Microstructural Evolution of HEAs Synthesized through the Melting and Casting Route
| HEA Composition | Processing Method | Observed Phase(s) | Microstructures and Comments | Reference |
|---|---|---|---|---|
| AlCoCrFeNi | Arc-melting | BCC | A dendritic structure is included. | [16][18][26,28] |
| AlTiVCr | Arc-melting | Single phase consisting of a B2 phase and a disordered BCC phase | The B2 phase is more stable than the disordered BCC phase. | [23][33] |
| AlCoFeNiTi | Arc-melting | BCC | A dendritic structure is included. | [24][34] |
| TiVZrNbHf | Arc-melting | Single-phase BCC | [25][35] | |
| AlCrFeNiMo0.2 | Vacuum Induction | BCC and B2 structure | The BCC phase is FeCrMo-rich, while the B2 phase is a NiAl-rich intermetallic compound. | [26][36] |
| NbCrMoTiAl0.5 | Arc-melting | Simple BCC | Mo segregates to the dendritic region. | [27][37] |
| NbCrMoTiVAl0.5Si0.3 | Cr, Ti, Al, and Si segregate to the interdendritic regions. | |||
| AlxCoFeNiSi (x > 0.3) | Arc-melting | BCC | [28][38] | |
| MoNbTaVW | Arc-melting | Single BCC | Dendritic and interdendritic regions are present due to constitutional segregation during solidification. | [29][30][39,40] |
| AlxCrFeMnNi0.5 | Arc-melting | BCC | [31][41] | |
| (x = 0.8–1.2) | ||||
| Nb25Mo25Ta25W25 | Arc-melting | BCC phase | There is no dendritic segregation. | [8][15] |
| Fe36Mn21Cr18Ni15Al10 | Arc-melting | Dual-phase 2 BCCs/B2 | The matrix phase (BCC) is rich in Fe and Cr. | [32][42] |
| The B2 phase is rich in Ni and Al. | ||||
| CoCrCuFeNi | Arc-melting | FCC | The interface morphology would grow in planar, cellular, and dendrite if the solidification rate is increased. | [33][34][18,43] |
| CoCrFeNiV0.5Cx | Arc-melting | FCC | A large number of M7C3-type interstitial carbides are formed at an annealing temperature of 700 °C and above. | [35][44] |
| (x = 0.01, 0.02, 0.03, and 0.04) | ||||
| Fe40Mn40Co10 |
3. Microstructural Evolution of HEAs Fabricated by Additive Manufacturing (AM)
| HEA Composition | Processing Method | Observed Phase(s) | Microstructures and Comments | Reference | |||||
|---|---|---|---|---|---|---|---|---|---|
| CoCrFeMnNi | Laser 3D printing | FCC (major) + BCC | An equiaxed-to-columnar transition structure was discovered in the melt pool. | [101][137] | |||||
| CoCrFeMnNi | Laser powder bed fusion (LPBF) | FCC + σ phase | Nanotwins were present in the printed sample. | [102][146] | |||||
| Mn segregates at the boundary of the weld pool due to its volatility. | |||||||||
| CoCrFeMnNi | Laser directed energy deposition | FCC solid solution | No phase transformation occurred | [103][147] | |||||
| Lattice strain and grain refinement occurred. | |||||||||
| AlCrFeCoNi | Selective electron beam melting (SEBM) | FCC + BCC | Phase evolution occurred during the preheating process | [97][98][134,135] | |||||
| AlCrFeCoMnNi | LPBF | BCC (B2, A2) | B2 (Ni-Al rich) and A2 (Fe-Cr rich) | [104][136] | |||||
| Due to liquid-phase spinodal decomposition and cubic nature of the HEA | |||||||||
| Al0.3CoCrFeNi | LPBF | Supersaturated FCC phase | Fine columnar grains were present due to rapid solidification and anisotropic heat removal. | [105][139] | |||||
| AlCoCrFeNiTi0.5 | Laser-engineered net shaping (LENS) | 2 BCC (B2, A2) | A fully equiaxed grain microstructure was exhibited rather than a columnar microstructure associated with alloys fabricated with AM. | [106][140] | |||||
| AlCrCuFeNi | LPBF | 2 BCC (B2, A2) | Unique columnar grains were present containing multiple ultrafine sub-grain structures. | [107][145] | |||||
| AlCrFeNiV | LPBF | FCC | Rapid cooling rate and solidification resulted in the formation of sub-grains in every columnar grain and L12 nano-phase. | [108][148] | |||||
| AlCrFe2Ni2 | LPBF | BCC | Columnar BCC of spinodal decomposed B2 and A2 structures was exhibited. | [109][149] | |||||
| Cr | |||||||||
| Cracks were present at the intergranular site. | |||||||||
| FeCoCrNi | LPBF | FCC | After annealing at 1373 K, columnar grains and equiaxial grains were found to co-exist. | [110][150] | |||||
| AlCoCrFeNi | Direct laser fabrication (DLF) | BCC (B2) | Intergranular needle-like and plate-like FCC phase precipitates and wall-shaped FCC phase precipitates were present along grain boundaries after aging at 800, 1000, and 1200 °C. | [111][151] | |||||
| MoNbTaW | Direct energy deposition (DED) | BCC | [112][152] | 10 | Vacuum induction | FCC | [ | ||
| Al0.5Cr1.0Mo1.0Nb | 36 | ] | [ | 45] | |||||
| 1.0Ta0.5 | SEBM | BCC | Two phases were present: TaMoNbCr and (TaMoNbCr)Al solid solutions. | [113][153] | CrMnFeCoNi | Arc-melting, Vacuum Induction | FCC | Precipitates of M23C6 and the σ phase exist following prolonged exposure at 700 °C. | [7] |
| CoCrCuFeNiAl | [ | 37 | ] | [11, | LENS | 46] | |||
| BCC (B2, A2) | Dendritic grains were present. | [ | 114 | ][115][154,155] | AlxCoCrFeNi | Arc-melting | FCC | The FCC phase is transformed to the BCC phase with the presence of a transition duplex FCC/BCC region as Al increases. | [38][47] |
| An ordered interface transition region was present between the two phases. | (x = 0–0.65) | ||||||||
| AlCoCrFeNi2.1 | LENS | Ordered FCC (L12) + BCC | Co, Cr, and Fe stabilize L12. | [116][156] | CoCrFeNiTi0.3 | Arc-melting | FCC | A crystalline structure is present consisting of a mixture of a (Ni, Ti)-rich R phase and a (Cr, Fe)-rich σ phase within the FCC matrix. | [39][48] |
| L12 and BCC are rich in nickel. | Al0.5CoCrCu0.5FeNi | Arc-melting | FCC | The BCC phase will evolve from the FCC phase with an increase in the Al content. | |||||
| Fe38.5Mn20Co20Cr15Si | [ | 40 | ] | [49] | |||||
| FCC + BCC duplex phases will evolve at Al (0.5–1.5). | |||||||||
| 5Cu1.5 | LPBF | FCC | Deformation-induced phase transformation of γ (FCC) to ε (HCP) occurred in the vicinity of microcracks. | [117][157] | |||||
| CoCrFeNi | 3D extrusion printing | FCC | There was complex structural evolution, from loosely packed oxide particles in the green body to fully-annealed, metallic CoCrFeNi. | CoCrFeNiNb0.25 | Arc-melting | FCC | Lath-shaped FCC precipitates + nano-basket-weave microstructures are randomly distributed in the proeutectic FCC phase. | [41][50] | |
| AlxCoCrFeNiTiy | Arc-melting | FCC | The Al and Ti content strongly affects the phase and microstructure. | [42][51] | |||||
| [ | 118 | ] | [ | 158] | |||||
| AlCrFeMoVx (x = 0 to 1) | LENS | BCC | The high solubility of V offers a broad range of solid solution strengthening of a compositionally complex but structurally simple BC matrix. | [118][158] | |||||
| ZrTiVCrFeNi | LENS | C14 Laves phase (major) + α-Ti solid solution | The C14 Laves phase becomes stable on exposure to annealing and hydrogen influence. | [119][159] | Co1.5CrFeNi1.5Ti0.5Mox | Arc-melting | FCC | An interdendritic phase, (Ni, Ti)-rich phase and dendritic (Fe, Cr)-rich phase are present when x = 0, 0.1. | [43][52] |
| 6FeNiCoSiCrAlTi | Laser cladding | BCC | Equiaxed polygonal grains, discontinuous interdendritic segregation, and nano-precipitates are present. | [120][160] | (x = 0, 0.1) | ||||
| MoFeCrTiW | Laser cladding | BCC | Cellular crystals are formed on which dispersion precipitates exist. | [121][161] | Mn22.3Fe22.2Ni22.2Ge16.65Si16.65 | Arc-melting | FCC | Magneto-structural first-order phase transition is exhibited. | [44][53] |
| TiZrNbMoV | LENS | FCC (δTiHx-type) + BCC (NbH∼0.4–type) | αZr-rich precipitates are present, in addition to the phases formed. | [122][162] | AlCrFeMnNi | Arc-melting | BCC (B2) + FCC | The BCC phase is interdendritic and rich in Al + Ni. | |
| Al0.5FeCu0.7 | [ | 45 | ] | [54] | |||||
| NiCoCr | Laser cladding | FCC + BCC + Al phases | A laser rapid cooling rate facilitates the formation of a simple structure and prohibits the formation of undesired intermetallic compounds. | [123][163] | Ni30Co30Cr10Fe10Al18W2 | Arc-melting | FCC + BCC | Fine, regular, lamellar eutectic + coarse irregular eutectic hierarchical microstructures are present. | [ |
| TiZrNbHfTa | Laser metal deposition (LMD) | 46 | ] | BCC[55 | An equiaxed grain shape is present.] | ||||
| [ | 124 | ] | [ | 164] | Al0.5CrFeMnNi0.5 | Arc-melting | FCC + BCC | A dendritic region (higher Al and Cr) and an interdendritic region are present. | [47][56] |
| Al0.5CrMoNbTa0.5 | Electron beam melting (EBM) | BCC | Intermetallic phases C14, C36, C15, and 6H are present. | [125][165] | Precipitates (AlNi B2 compound) are present. | ||||
| Ni6Cr4WFe9Ti | LPBF | FCC | Tiny precipitates of an unknown phase are present. | [126][166] | AlxCoCrFeNi | Arc-melting | FCC + BCC | An AlNi-rich precipitate is formed. | |
| FeCoCrNiC0.05 | LPBF | FCC | Nano-scale Cr23C6-type carbides can precipitate under annealing conditions. | [48][57] | |||||
| [ | 127 | ] | [ | 167 | (x = 0.45–0.85) | ||||
| ] | Cr2Cu2FeNi2Mn2 | Arc-melting | FCC + BCC | A dendritic and interdendritic phase is present. | [49][50][58,59] | ||||
| Cr2Cu2NiMn2 | |||||||||
| CrCu2Fe2NiMn | Cu, Mn, Cr, and Fe are segregated in dendritic/interdendritic regions, while Ni is homogeneously distributed in the alloy. | ||||||||
| Cr2CuFe2NiMn | |||||||||
| Alx(CoCrFeMnNi)100−x | Arc-melting | FCC + BCC | An increase in Al turns the dendritic structure to a lamella-like structure, hence the transit from the FCC to the BCC phase. | [51][60] | |||||
| CoCrFeMnNiZrx (x = 0–0.3) | Arc-melting | FCC + BCC | Dendritic and interdendritic regions are present. | [52][61] | |||||
| The interdendritic region increases with an increase in the Zr content. | |||||||||
| AlCoCrCuxNiTi | Arc-melting | FCC + BCC | Dendritic (contains compound impurities) and chrysanthemum-shape dendrites are present. | [53][62] | |||||
| (x = 0.5–0.8) | Cu segregates in the interdendritic region. | ||||||||
| CoCuyFeNiTix | Arc-melting | 2 FCCs + BCC | FCC 1 is Cu rich, and FCC 2 is Co rich (x = 1/3, 3/7, and 3/5). | [54][63] | |||||
| The BCC phase is β Ti rich (x = 3/5). | |||||||||
| CoCrFeNiCuAl | Arc-melting | FCC + BCC | A cast-dendritic morphology is present. | [55][56][64,65] | |||||
| The BCC phase is an ordered one. | |||||||||
| of 2 FCC phases are present. | |||||||||
| Fe50-XMn30Co10Cr10BX | Arc-melting | FCC + BCC | The addition of boron promotes the formation of M2B-type borides (M = Cr, Fe). | [57][66] | |||||
| (x = 0, 0.3, 0.6, 1.7 wt%) | |||||||||
| AlCrCuFeMnNi | Vacuum Induction | 2 BCCs (B2 + A2) + FCC | The 2BCC phase is formed by spinodal decomposition, i.e., B2 (NiAl dendrite matrix) and A2 (Cr-Fe rich) embedded precipitate. | [58][67] | |||||
| Al0.5CoCrFeNi | Arc-melting, Vacuum Induction | FCC + BCC crystalline structures | The presence of the Al-Ni-rich phase decreases as the aging temperature increases and, hence, leads to an increase in the amount of Al-(Ni, Co, Cr, Fe). | [59][60][68,69] | |||||
| NbMoTaTi–(W, V) | Arc-melting | BCC + HCP—with W inclusion | The HEA with “V” shows a dendritic/cellular microstructure rich in Ti and V. | [61][70] | |||||
| BCC—with V inclusion | The HEA with “W” forms a Ti-rich HCP phase. | ||||||||
| Al0.5CrCuNiV | Arc-melting | FCC + 2 BCCs + B2 | A dendrite rich in Cr and V is present. | [62][71] | |||||
| The incorporation of Cu into the 2-BBC phase differentiates it from the B2 phase. | |||||||||
| AlCoCrFeNi2.1 | Vacuum Induction | Dual-phase FCC + BCC (B2) | - | [63][72] | |||||
| AlCrCuFeNi | Arc-melting | FCC + BCC | The content of Ni has a significant effect on the HEA microstructure. | [64][65][73,74] |
4. Recommendations for Future Studies
Large differences in the melting temperatures of the constituent elements due to compositional complexity result in elemental segregation, dendritic structure, and residual stress in HEAs fabricated using the melting and casting route. To address these discrepancies, the rate of cooling, differences in the local atomic arrangement, and the varying elemental diffusivity must be taken into consideration in future studies. Faster cooling routes such as suction casting, injection casting, melt spinning, or splat cooling suppress the precipitation of the secondary phase and thereby form a predominantly stable single-phase structure. Hence, induction remelting can reduce microsegregation, reduce the inhomogeneity challenge, and refine the grain size.
Most of the studies on HEAs fabricated by MA are focused on varying the milling duration in achieving a homogeneous solid solution of the elements. However, since the parameters of the MA process are not independent of each other, it is imperative to know that other parameters such as milling speed, the BPR, grinding media, and the milling environment are given some attention in future studies. These other parameters also significantly influence the heat generated during milling and the diffusion of elements in the solid solution process. A lower sintering temperature (depends on the melting temperatures of constituent elements) should also be considered.
There is no adequate information to better understand how, where, and why voids and porosity were formed in most AM-fabricated materials. More attention is needed in this area as controlling their distribution or avoiding them is crucial and requires a better understanding; hence, these defects are undesirable in certain engineering applications. Therefore, there is a need for the development and standardization of economically viable and printable materials for engineering applications in the AM fabrication technique spectrum to complement its processing advantages. Urgent attention is needed in developing computer-aided design tools and predictive models of both the printing process and the post-printing material properties in future studies.
[128][200], and more researchers have used the combinatorial approach in processing multicomponent alloys, more attention is still needed on this method owing to the possibility of exploring composition space. Thus, observations suggest that proper selection of the chemical composition and an appropriate processing route combined with appropriate thermomechanical treatment may offer an opportunity to manipulate the strengthening mechanism to enhance HEAs’ mechanical properties. An optimal composition with required properties could be more efficient. Therefore, more research with modeling and simulations is required, in addition to computational tools and integrated computational material engineering available.