Polyhydroxyalkanoates (PHAs) constitute a family of biopolyesters that are synthesized and accumulate within the cellular structure of prokaryotic cells by bacteria, and they act as carbon and energy reserve materials under conditions of limited nutrient, such as nitrogen, oxygen, phosphorous or magnesium. As naturally derived materials, PHAs have been used for multiple cell and tissue engineering applications; however, their widespread biomedical applications are limited due to their lack of toughness, elasticity, hydrophilicity and bioactivity. To overcome this challenge combination of PHA with different polymers and inorganic materials are used to form hybrid composites with improved structural and functional properties. This work presents an up-to-date overview of ongoing research strategies that make use of PHAs as versatile and prospective biomaterials.
Polyhydroxyalkanoates (PHAs) constitute a family of biopolyesters that are synthesized and accumulate within the cellular structure of prokaryotic cells by bacteria, and they act as carbon and energy reserve materials under conditions of limited nutrient, such as nitrogen, oxygen, phosphorous or magnesium. As naturally derived materials, PHAs have been used for multiple cell and tissue engineering applications; however, their widespread biomedical applications are limited due to their lack of toughness, elasticity, hydrophilicity and bioactivity. To overcome this challenge combination of PHA with different polymers and inorganic materials are used to form hybrid composites with improved structural and functional properties.
- polyhydroxyalkanoates
- biopolymers
- biocompatibility
- biodegradibility
1. Introduction
2. The Most Important Properties of the Hybrids Based on PHAs
2.1. Wettability of the Composites
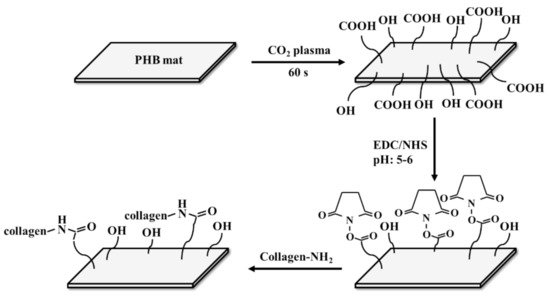
2.2. Physico-Mechanical Properties
References
- Favaro, L.; Basaglia, M.; Casella, S. Improving polyhydroxyalkanoate production from inexpensive carbon sources by genetic approaches: A review. Biofuels Bioprod. Biorefining 2019, 13, 208–227.
- Saratale, R.G.; Cho, S.-K.; Saratale, G.D.; Kadam, A.A.; Ghodake, G.S.; Kumar, M.; Mulla, S.I. A comprehensive overview and recent advances on polyhydroxyalkanoates (PHA) production using various organic waste streams. Bioresour. Technol. 2021, 325, 124685.
- Tan, D.; Wang, Y.; Tong, Y.; Chen, G.-Q. Grand Challenges for Industrializing Polyhydroxyalkanoates (PHAs). Trends Biotechnol. 2021.
- Akaraonye, E.; Keshavarz, T.; Roy, I. Production of polyhydroxyalkanoates: The future green materials of choice. J. Chem. Technol. Biotechnol. 2010, 85, 732–743.
- Saratale, R.G.; Saratale, G.D.; Cho, S.K.; Kim, D.S.; Ghodake, G.S.; Kadam, A.; Kumar, G.; Bharagava, R.N.; Banu, R.; Shin, H.S. Pretreatment of kenaf (Hibiscus cannabinus L.) biomass feedstock for polyhydroxybutyrate (PHB) production and characterization. Bioresour. Technol. 2019, 282, 75–80.
- Sirohi, R.; Pandey, J.P.; Gaur, V.K.; Gnansounou, E.; Sindhu, R. Critical overview of biomass feedstocks as sustainable substrates for the production of polyhydroxybutyrate (PHB). Bioresour. Technol. 2020, 311, 123536.
- Hazer, D.B.; Kılıçay, E.; Hazer, B. Poly(3-hydroxyalkanoate)s: Diversification and biomedical applications: A state of the art review. Mater. Sci. Eng. C 2012, 32, 637–647.
- Tian, H.; Tang, Z.; Zhuang, X.; Chen, X.; Jing, X. Biodegradable synthetic polymers: Preparation, functionalization and biomedical application. Prog. Polym. Sci. 2012, 37, 237–280.
- Bose, S.; Roy, M.; Bandyopadhyay, A. Recent advances in bone tissue engineering scaffolds. Trends Biotechnol. 2012, 30, 546–554.
- Anjum, A.; Zuber, M.; Zia, K.M.; Noreen, A.; Anjum, M.N.; Tabasum, S. Microbial production of polyhydroxyalkanoates (PHAs) and its copolymers: A review of recent advancements. Int. J. Biol. Macromol. 2016, 89, 161–174.
- Zine, R.; Sinha, M. Nanofibrous poly (3-hydroxybutyrate-co-3-hydroxyvalerate)/collagen/graphene oxide scaffolds for wound coverage. Mater. Sci. Eng. C 2017, 80, 129–134.
- Yang, H.-X.; Sun, M.; Zhang, Y.; Zhou, P. Degradable PHBHHx modified by the silk fibroin for the applications of cardiovascular tissue engineering. ISRN Mater. Sci. 2011, 2011, 1–11.
- Heidari-Keshel, S.; Ahmadian, M.; Biazar, E.; Gazmeh, A.; Rabiei, M.; Adibi, M.; Shabani, M. Surface modification of Poly Hydroxybutyrate (PHB) nanofibrous mat by collagen protein and its cellular study. Mater. Technol. 2016, 31, 799–805.
- Prabhakaran, M.P.; Vatankhah, E.; Ramakrishna, S. Electrospun aligned PHBV/collagen nanofibers as substrates for nerve tissue engineering. Biotechnol. Bioeng. 2013, 110, 2775–2784.
- Chiulan, I.; Mihaela Panaitescu, D.; Nicoleta Frone, A.; Teodorescu, M.; Andi Nicolae, C.; Căşărică, A.; Sălăgeanu, A. Biocompatible polyhydroxyalkanoates/bacterial cellulose composites: Preparation, characterization, and in vitro evaluation. J. Biomed. Mater. Res. Part A 2016, 104, 2576–2584.
- Zarei, M.; Karbasi, S. Evaluation of the effects of multiwalled carbon nanotubes on electrospun poly (3-hydroxybutirate) scaffold for tissue engineering applications. J. Porous Mater. 2018, 25, 259–272.
- Tesema, Y.; Raghavan, D.; Stubbs, J., III. Bone cell viability on collagen immobilized poly (3-hydroxybutrate-co-3-hydroxyvalerate) membrane: Effect of surface chemistry. J. Appl. Polym. Sci. 2004, 93, 2445–2453.
- Andreessen, B.; Taylor, N.; Steinbüchel, A. Poly (3-hydroxypropionate): A promising alternative to fossil fuel-based materials. Appl. Environ. Microbiol. 2014, 80, 6574–6582.
- Aeschelmann, F.; Carus, M. Biobased building blocks and polymers in the world: Capacities, production, and applications—Status quo and trends towards 2020. Ind. Biotechnol. 2015, 11, 154–159.
- Market, M.A. Polyhydroxyalkanoate (PHA) Market by Type (Short Chain Length, Medium Chain Length), Production Method (Sugar Fermentation, Vegetable Oil Fermentation, Methane Fermentation), Application, and Region-Global Forecast to 2024. Markets and Markets Research Private Ltd., 2019. Available online: (accessed on 18 December 2017).
- Saratale, G.D.; Bhosale, R.; Pugazendhi, A.; Mahmoud, E.; Sirohi, R.; Bhatia, S.K.; Shin, H.S. A review on valorization of spent coffee grounds (SCG) towards biopolymers and biocatalysts production. Bioresour. Technol. 2020, 314, 123800.
- Saratale, G.D.; Saratale, R.G.; Varjani, S.; Cho, S.-K.; Ghodake, G.S.; Kadam, A. Development of ultrasound aided chemical pretreatment methods to enrich saccharification of wheat waste biomass for polyhydroxybutyrate production and its characterization. Ind. Crop. Prod. 2020, 150, 112425.
- Saratale, R.G.; Cho, S.-K.; Ghodake, G.S.; Shin, H.-S.; Saratale, G.D.; Park, Y.; Lee, H.-S.; Bharagava, R.N.; Kim, D.-S. Utilization of noxious weed water hyacinth biomass as a potential feedstock for biopolymers production: A novel approach. Polymers 2020, 12, 1704.
- Sultana, N.; Khan, T.H. In vitro degradation of PHBV scaffolds and nHA/PHBV composite scaffolds containing hydroxyapatite nanoparticles for bone tissue engineering. J. Nanomater. 2012, 2012, 1–12.
- Kumarasuriyar, A.; Jackson, R.A.; Grøndahl, L.; Trau, M.; Nurcombe, V.; Cool, S.M. Poly (β-hydroxybutyrate-co-β-hydroxyvalerate) supports in vitro osteogenesis. Tissue Eng. 2005, 11, 1281–1295.
- Wang, Y.; Gao, R.; Wang, P.-P.; Jian, J.; Jiang, X.-L.; Yan, C.; Lin, X.; Wu, L.; Chen, G.-Q.; Wu, Q. The differential effects of aligned electrospun PHBHHx fibers on adipogenic and osteogenic potential of MSCs through the regulation of PPARγ signaling. Biomaterials 2012, 33, 485–493.
- Wang, Y.; Bian, Y.-Z.; Wu, Q.; Chen, G.-Q. Evaluation of three-dimensional scaffolds prepared from poly (3-hydroxybutyrate-co-3-hydroxyhexanoate) for growth of allogeneic chondrocytes for cartilage repair in rabbits. Biomaterials 2008, 29, 2858–2868.
- Goonoo, N.; Bhaw-Luximon, A.; Passanha, P.; Esteves, S.R.; Jhurry, D. Third generation poly (hydroxyacid) composite scaffolds for tissue engineering. J. Biomed. Mater. Res. Part B Appl. Biomater. 2017, 105, 1667–1684.
- Cheng, S.; Wu, Q.; Yang, F.; Xu, M.; Leski, M.; Chen, G.-Q. Influence of DL-β-hydroxybutyric acid on cell proliferation and calcium influx. Biomacromolecules 2005, 6, 593–597.
- Wang, Y.-W.; Wu, Q.; Chen, G.-Q. Attachment, proliferation and differentiation of osteoblasts on random biopolyester poly (3-hydroxybutyrate-co-3-hydroxyhexanoate) scaffolds. Biomaterials 2004, 25, 669–675.
- Ang, S.L.; Shaharuddin, B.; Chuah, J.-A.; Sudesh, K. Electrospun poly (3-hydroxybutyrate-co-3-hydroxyhexanoate)/silk fibroin film is a promising scaffold for bone tissue engineering. Int. J. Biol. Macromol. 2020, 145, 173–188.
- Sadeghi, D.; Karbasi, S.; Razavi, S.; Mohammadi, S.; Shokrgozar, M.A.; Bonakdar, S. Electrospun poly (hydroxybutyrate)/chitosan blend fibrous scaffolds for cartilage tissue engineering. J. Appl. Polym. Sci. 2016, 133.
- Shishatskaya, E.I.; Khlusov, I.A.; Volova, T.G. A hybrid PHB–hydroxyapatite composite for biomedical application: Production, in vitro and in vivo investigation. J. Biomater. Sci. Polym. Ed. 2006, 17, 481–498.
- Ding, Y.; Roether, J.A.; Boccaccini, A.R.; Schubert, D.W. Fabrication of electrospun poly (3-hydroxybutyrate)/poly (ε-caprolactone)/silica hybrid fibermats with and without calcium addition. Eur. Polym. J. 2014, 55, 222–234.
- Misra, S.K.; Mohn, D.; Brunner, T.J.; Stark, W.J.; Philip, S.E.; Roy, I.; Salih, V.; Knowles, J.C.; Boccaccini, A.R. Comparison of nanoscale and microscale bioactive glass on the properties of P (3HB)/Bioglass® composites. Biomaterials 2008, 29, 1750–1761.
- Sabapathy, P.C.; Devaraj, S.; Meixner, K.; Anburajan, P.; Kathirvel, P.; Ravikumar, Y.; Qi, X. Recent developments in Polyhydroxyalkanoates (PHAs) production—A review. Bioresour. Technol. 2020, 306, 123132.
- Pagliano, G.; Galletti, P.; Samorì, C.; Zaghini, A.; Torri, C. Recovery of Polyhydroxyalkanoates From Single and Mixed Microbial Cultures: A Review. Front. Bioeng. Biotechnol. 2021, 9, 54.
- Giubilini, A.; Bondioli, F.; Messori, M.; Nyström, G.; Siqueira, G. Advantages of Additive Manufacturing for Biomedical Applications of Polyhydroxyalkanoates. Bioengineering 2021, 8, 29.
- Tarrahi, R.; Fathi, Z.; Seydibeyoğlu, M.Ö.; Doustkhah, E.; Khataee, A. Polyhydroxyalkanoates (PHA): From production to nanoarchitecture. Int. J. Biol. Macromol. 2020, 146, 596–619.
- Ang, S.L.; Sivashankari, R.; Shaharuddin, B.; Chuah, J.-A.; Tsuge, T.; Abe, H.; Sudesh, K. Potential Applications of Polyhydroxyalkanoates as a Biomaterial for the Aging Population. Polym. Degrad. Stab. 2020, 181, 109371.
- Chuenjitkuntaworn, B.; Inrung, W.; Damrongsri, D.; Mekaapiruk, K.; Supaphol, P.; Pavasant, P. Polycaprolactone/hydroxyapatite composite scaffolds: Preparation, characterization, and in vitro and in vivo biological responses of human primary bone cells. J. Biomed. Mater. Res. Part A 2010, 94, 241–251.
- Guzmán, D.; Kirsebom, H.; Solano, C.; Quillaguamán, J.; Hatti-Kaul, R. Preparation of hydrophilic poly (3-hydroxybutyrate) macroporous scaffolds through enzyme-mediated modifications. J. Bioact. Compat. Polym. 2011, 26, 452–463.
- Köse, G.T.; Korkusuz, F.; Korkusuz, P.; Purali, N.; Özkul, A.; Hasırcı, V. Bone generation on PHBV matrices: An in vitro study. Biomaterials 2003, 24, 4999–5007.
- Wang, N.; Zhou, Z.; Xia, L.; Dai, Y.; Liu, H. Fabrication and characterization of bioactive β-Ca2SiO4/PHBV composite scaffolds. Mater. Sci. Eng. C 2013, 33, 2294–2301.
- Sousa, I.; Mendes, A.; Pereira, R.F.; Bártolo, P.J. Collagen surface modified poly (ε-caprolactone) scaffolds with improved hydrophilicity and cell adhesion properties. Mater. Lett. 2014, 134, 263–267.
- Kim, M.S.; Na Shin, Y.; Cho, M.H.; Kim, S.H.; Kim, S.K.; Cho, Y.H.; Khang, G.; Lee, I.W.; Lee, H.B. Adhesion behavior of human bone marrow stromal cells on differentially wettable polymer surfaces. Tissue Eng. 2007, 13, 2095–2103.
- Lee, J.H.; Khang, G.; Lee, H.B. Interaction of cells on chargeable functional group gradient surfaces. Biomaterials 1997, 18, 351–358.
- Misra, S.K.; Nazhat, S.N.; Valappil, S.P.; Moshrefi-Torbati, M.; Wood, R.J.K.; Roy, I.; Boccaccini, A.R. Fabrication and characterization of biodegradable poly (3-hydroxybutyrate) composite containing bioglass. Biomacromolecules 2007, 8, 2112–2119.
- Ishaug-Riley, S.L.; Okun, L.E.; Prado, G.; Applegate, M.A.; Ratcliffe, A. Human articular chondrocyte adhesion and proliferation on synthetic biodegradable polymer films. Biomaterials 1999, 20, 2245–2256.
- Karahaliloğlu, Z. Cell-compatible PHB/silk fibroin composite nanofiber mat for tissue engineering applications. Turk. J. Biol. 2017, 41, 503–513.
- Lei, C.; Zhu, H.; Li, J.; Li, J.; Feng, X.; Chen, J. Preparation and characterization of polyhydroxybutyrate-co-hydroxyvalerate/silk fibroin nanofibrous scaffolds for skin tissue engineering. Polym. Eng. Sci. 2014, 55, 907–916.
- Karbasi, S.; Karimi, A.; Razavi, S.; Zargar, E.N. Poly (hydroxybutyrate)/chitosan aligned electrospun scaffold as a novel substrate for nerve tissue engineering. Adv. Biomed. Res. 2018, 7, 44.
- Hu, S.-G.; Jou, C.-H.; Yang, M.-C. Biocompatibility and antibacterial activity of chitosan and collagen immobilized poly (3-hydroxybutyric acid-co-3-hydroxyvaleric acid). Carbohydr. Polym. 2004, 58, 173–179.
- Mohammadalizadeh, Z.; Karbasi, S.; Arasteh, S. Physical, mechanical and biological evaluation of poly (3-hydroxybutyrate)-chitosan/MWNTs as a novel electrospun scaffold for cartilage tissue engineering applications. Polym. Plast. Technol. Mater. 2020, 59, 417–429.
- Foroughi, M.R.; Karbasi, S.; Khoroushi, M.; Khademi, A.A. Polyhydroxybutyrate/chitosan/bioglass nanocomposite as a novel electrospun scaffold: Fabrication and characterization. J. Porous Mater. 2017, 24, 1447–1460.
- Zhou, H.; Lee, J. Nanoscale hydroxyapatite particles for bone tissue engineering. Acta Biomater. 2011, 7, 2769–2781.
- Ito, Y.; Hasuda, H.; Kamitakahara, M.; Ohtsuki, C.; Tanihara, M.; Kang, I.-K.; Kwon, O.H. A composite of hydroxyapatite with electrospun biodegradable nanofibers as a tissue engineering material. J. Biosci. Bioeng. 2005, 100, 43–49.
- Ramier, J.; Bouderlique, T.; Stoilova, O.; Manolova, N.; Rashkov, I.; Langlois, V. Biocomposite scaffolds based on electrospun poly (3-hydroxybutyrate) nanofibers and electrosprayed hydroxyapatite nanoparticles for bone tissue engineering applications. Mater. Sci. Eng. C 2014, 38, 161–169.
- Wu, T.-J.; Huang, H.-H.; Lan, C.-W.; Lin, C.-H.; Hsu, F.-Y.; Wang, Y.-J. Studies on the microspheres comprised of reconstituted collagen and hydroxyapatite. Biomaterials 2004, 25, 651–658.
- Tachibana, A.; Kaneko, S.; Tanabe, T.; Yamauchi, K. Rapid fabrication of keratin–hydroxyapatite hybrid sponges toward osteoblast cultivation and differentiation. Biomaterials 2005, 26, 297–302.
- Hosoya, K.; Ohtsuki, C.; Kawai, T.; Kamitakahara, M.; Ogata, S.-i.; Miyazaki, T. A novel covalently crosslinked gel of alginate and silane with the ability to form bone-like apatite. J. Biomed. Mater. Res. Part A 2004, 71, 596–601.
- Furuzono, T.; Wang, P.-L.; Korematsu, A.; Miyazaki, K.; Oido-Mori, M.; Kowashi, Y.; Ohura, K.; Tanaka, J.; Kishida, A. Physical and biological evaluations of sintered hydroxyapatite/silicone composite with covalent bonding for a percutaneous implant material. J. Biomed. Mater. Res. Part B 2003, 65, 217–226.
- Chou, Y.-F.; Huang, W.; Dunn, J.C.; A Miller, T.; Wu, B.M. The effect of biomimetic apatite structure on osteoblast viability, proliferation, and gene expression. Biomaterials 2005, 26, 285–295.
- Lin, X.; Yin, M.; Liu, Y.; Li, L.; Ren, X.; Sun, Y.; Huang, T.-S. Biodegradable polyhydroxybutyrate/poly-ε-caprolactone fibrous membranes modified by silica composite hydrol for super hydrophobic and outstanding antibacterial application. J. Ind. Eng. Chem. 2018, 63, 303–311.
- Rivera-Briso, A.L.; Aachmann, F.L.; Moreno-Manzano, V.; Serrano-Aroca, A. Graphene oxide nanosheets versus carbon nanofibers: Enhancement of physical and biological properties of poly (3-hydroxybutyrate-co-3-hydroxyvalerate) films for biomedical applications. Int. J. Biol. Macromol. 2020, 143, 1000–1008.
- Du, Z.; Jia, S.; Xiong, P.; Cai, Z. Preparation of protein nanoparticle-coated poly (hydroxybutyrate) electrospun nanofiber based scaffold for biomedical applications. Int. J. Polym. Mater. Polym. Biomater. 2021, 1–15.
- Zarei, M.; Karbasi, S.; Aslani, F.S.; Zare, S.; Koohi-Hosseinabad, O.; Tanideh, N. In Vitro and In Vivo Evaluation of Poly (3-hydroxybutyrate)/Carbon Nanotubes Electrospun Scaffolds for Periodontal Ligament Tissue Engineering. J. Dent. 2020, 21, 18.
- Zhijiang, C.; Cong, Z.; Jie, G.; Qing, Z.; Kongyin, Z. Electrospun carboxyl multi-walled carbon nanotubes grafted polyhydroxybutyrate composite nanofibers membrane scaffolds: Preparation, characterization and cytocompatibility. Mater. Sci. Eng. C 2018, 82, 29–40.
- Guo, J.; Zhang, Q.; Cai, Z.; Zhao, K. Preparation and dye filtration property of electrospun polyhydroxybutyrate–calcium alginate/carbon nanotubes composite nanofibrous filtration membrane. Sep. Purif. Technol. 2016, 161, 69–79.
- Ai, J.; Heidari K, S.; Ghorbani, F.; Ejazi, F.; Biazar, E.; Asefnejad, A.; Montazeri, M. Fabrication of coated-collagen electrospun PHBV nanofiber film by plasma method and its cellular study. J. Nanomater. 2011.
- Yildirim, E.D.; Pappas, D.; Güçeri, S.; Sun, W. Enhanced cellular functions on polycaprolactone tissue scaffolds by O2 plasma surface modification. Plasma Process. Polym. 2011, 8, 256–267.
- Domingos, M.; Intranuovo, F.; Gloria, A.; Gristina, R.; Ambrosio, L.; Bártolo, P.J.; Favia, P. Improved osteoblast cell affinity on plasma-modified 3-D extruded PCL scaffolds. Acta Biomater. 2013, 9, 5997–6005.
- Bormashenko, E.; Chaniel, G.; Grynyov, R. Towards understanding hydrophobic recovery of plasma treated polymers: Storing in high polarity liquids suppresses hydrophobic recovery. Appl. Surf. Sci. 2013, 273, 549–553.
- Yang, H.; Sun, M.; Zhou, P.; Pan, L.; Wu, C. Silk fibroins modify the atmospheric low temperature plasma-treated poly (3-hydroxybutyrate-co-3-hydroxyhexanoate) film for the application of cardiovascular tissue engineering. J. Biomed. Sci. Eng. 2010, 3, 1146.
- Chen, J.-P.; Su, C.-H. Surface modification of electrospun PLLA nanofibers by plasma treatment and cationized gelatin immobilization for cartilage tissue engineering. Acta Biomater. 2011, 7, 234–243.
- Unalan, I.; Colpankan, O.; Albayrak, A.Z.; Gorgun, C.; Urkmez, A.S. Biocompatibility of plasma-treated poly (3-hydroxybutyrate-co-3-hydroxyvalerate) nanofiber mats modified by silk fibroin for bone tissue regeneration. Mater. Sci. Eng. C 2016, 68, 842–850.
- Zamanifard, M.; Khorasani, M.T.; Daliri, M.; Parvazinia, M. Preparation and modeling of electrospun polyhydroxybutyrate/polyaniline composite scaffold modified by plasma and printed by an inkjet method and its cellular study. J. Biomater. Sci. Polym. Ed. 2020, 31, 1515–1537.
- Reignier, J.; Huneault, M.A. Preparation of interconnected poly (ε-caprolactone) porous scaffolds by a combination of polymer and salt particulate leaching. Polymer 2006, 47, 4703–4717.
- Hutmacher, D.W. Scaffolds in tissue engineering bone and cartilage. Biomaterials 2000, 21, 2529–2543.
- Tai, H.-Y.; Fu, E.; Cheng, L.-P.; Don, T.-M. Fabrication of asymmetric membranes from polyhydroxybutyrate and biphasic calcium phosphate/chitosan for guided bone regeneration. J. Polym. Res. 2014, 21, 421.
- Tanideh, N.; Azarpira, N.; Sarafraz, N.; Zare, S.; Rowshanghiyas, A.; Farshidfar, N.; El Fray, M. Poly (3-Hydroxybutyrate)-Multiwalled Carbon Nanotubes Electrospun Scaffolds Modified with Curcumin. Polymers 2020, 12, 2588.
- Wang, Y.-W.; Wu, Q.; Chen, J.; Chen, G.-Q. Evaluation of three-dimensional scaffolds made of blends of hydroxyapatite and poly (3-hydroxybutyrate-co-3-hydroxyhexanoate) for bone reconstruction. Biomaterials 2005, 26, 899–904.
- Hayati, A.N.; Rezaie, H.R.; Hosseinalipour, S.M. Preparation of poly (3-hydroxybutyrate)/nano-hydroxyapatite composite scaffolds for bone tissue engineering. Mater. Lett. 2011, 65, 736–739.
- Kouhi, M.; Prabhakaran, M.P.; Shamanian, M.; Fathi, M.; Morshed, M.; Ramakrishna, S. Electrospun PHBV nanofibers containing HA and bredigite nanoparticles: Fabrication, characterization and evaluation of mechanical properties and bioactivity. Compos. Sci. Technol. 2015, 121, 115–122.
- Sadat-Shojai, M.; Khorasani, M.-T.; Jamshidi, A. A new strategy for fabrication of bone scaffolds using electrospun nano-HAp/PHB fibers and protein hydrogels. Chem. Eng. J. 2016, 289, 38–47.
- Hayati, A.N.; Hosseinalipour, S.; Rezaie, H.; Shokrgozar, M.A. Characterization of poly (3-hydroxybutyrate)/nano-hydroxyapatite composite scaffolds fabricated without the use of organic solvents for bone tissue engineering applications. Mater. Sci. Eng. C 2012, 32, 416–422.
- Chen, Z.; Song, Y.; Zhang, J.; Liu, W.; Cui, J.; Li, H. Laminated electrospun nHA/PHB-composite scaffolds mimicking bone extracellular matrix for bone tissue engineering. Mater. Sci. Eng. C 2017, 72, 341–351.
- Daranarong, D.; Chan, R.T.H.; Wanandy, N.S.; Molloy, R.; Punyodom, W.; Foster, L.J.R. Electrospun polyhydroxybutyrate and poly (L-lactide-co-ε-caprolactone) composites as nanofibrous scaffolds. BioMed Res. Int. 2014, 2014, 1–12.
- Manikandan, N.A.; Pakshirajan, K.; Pugazhenthi, G. Preparation and characterization of environmentally safe and highly biodegradable microbial polyhydroxybutyrate (PHB) based graphene nanocomposites for potential food packaging applications. Int. J. Biol. Macromol. 2020, 154, 866–877.
- Pramanik, N.; Bhattacharya, S.; Rath, T.; De, J.; Adhikary, A.; Basu, R.K.; Kundu, P.P. Polyhydroxybutyrate-co-hydroxyvalerate copolymer modified graphite oxide based 3D scaffold for tissue engineering application. Mater. Sci. Eng. C 2019, 94, 534–546.
- Bal, B.; Tugluca, I.B.; Koc, N.; Isoglu, I.A. On the detailed mechanical response investigation of PHBV/PCL and PHBV/PLGA electrospun mats. Mater. Res. Express 2019, 6, 065411.
- Diez-Pascual, A.M. Development and characterization of chitosan-grafted polycaprolactone/poly (3-hydroxybutyrate-co-3-hydroxyhexanoate) fiber blends for tissue engineering applications. Int. J. Comput. Methods Exp. Meas. 2017, 5, 713–722.
- Cai, Z.; Xiong, P.; He, S.; Zhu, C. Improved piezoelectric performances of highly orientated poly (β-hydroxybutyrate) electrospun nanofiber membrane scaffold blended with multiwalled carbon nanotubes. Mater. Lett. 2019, 240, 213–216.
- Ma, Y.; Zheng, Y.; Wei, G.; Song, W.; Hu, T.; Yang, H.; Xue, R. Processing, structure, and properties of multiwalled carbon nanotube/poly (hydroxybutyrate-co-valerate) biopolymer nanocomposites. J. Appl. Polym. Sci. 2012, 125, E620–E629.
- Liu, A.R.; Xu, B.Z.; Chen, C.C.; Huang, D.Y.; Liang, E.W.; Ge, F.X.; Ge, G.J. Effects of modified SWCNT on the mechanical and thermal properties of PLA/PHB bio-composites. AIP Adv. 2020, 10, 075122.
- Zhao, S.; Zhu, M.; Zhang, J.; Zhang, Y.; Liu, Z.; Zhu, Y.; Zhang, C. Three dimensionally printed mesoporous bioactive glass and poly (3-hydroxybutyrate-co-3-hydroxyhexanoate) composite scaffolds for bone regeneration. J. Mater. Chem. B 2014, 2, 6106–6118.
- Wu, J.; Wu, Z.; Xue, Z.; Li, H.; Liu, J. PHBV/bioglass composite scaffolds with co-cultures of endothelial cells and bone marrow stromal cells improve vascularization and osteogenesis for bone tissue engineering. RSC Adv. 2017, 7, 22197–22207.
- Yang, S.; Wang, J.; Tang, L.; Ao, H.; Tan, H.; Tang, T.; Liu, C. Mesoporous bioactive glass doped-poly (3-hydroxybutyrate-co-3-hydroxyhexanoate) composite scaffolds with 3-dimensionally hierarchical pore networks for bone regeneration. Colloids Surf. B Biointerfaces 2014, 116, 72–80.
- Ranjbar-Mohammadi, M.; Prabhakaran, M.P.; Bahrami, S.H.; Ramakrishna, S. Gum tragacanth/poly (l-lactic acid) nanofibrous scaffolds for application in regeneration of peripheral nerve damage. Carbohydr. Polym. 2016, 140, 104–112.
- Landel, R.F.; Nielsen, L.E. Mechanical Properties of Polymers and Composites; CRC Press: Boca Raton, FL, USA, 1993.
- Bleach, N.C.; Nazhat, S.N.; Tanner, K.E.; Kellomäki, M.; Törmälä, P. Effect of filler content on mechanical and dynamic mechanical properties of particulate biphasic calcium phosphate—Polylactide composites. Biomaterials 2002, 23, 1579–1585.
