Telocytes/CD34+ stromal cells in the normal and pathological peripheral nervous system (PNS). We consider the following aspects: (A) general characteristics of telocytes; (B) the presence, characteristics and arrangement of telocytes in the normal PNS, including i) nerve epi-perineurium and endoneurium (e.g., telopodes extending into the endoneurial space); ii) sensory nerve endings (e.g., Meissner and Pacinian corpuscles, and neuromuscular spindles); iii) ganglia; and iv) the intestinal autonomic nervous system; (C) the telocytes in the pathologic PNS, encompassing (i) hyperplastic neurogenic processes (neurogenic hyperplasia of the appendix and gallbladder), highly demonstrative of telocyte characteristics and relations, (ii) PNS tumours, such as neurofibroma, schwannoma and granular cell tumour.
We studied telocytes/CD34+ stromal cells in the normal and pathological peripheral nervous system (PNS). We consider the following aspects: (A) general characteristics of telocytes; (B) the presence, characteristics and arrangement of telocytes in the normal PNS, including i) nerve epi-perineurium and endoneurium (e.g., telopodes extending into the endoneurial space); ii) sensory nerve endings (e.g., Meissner and Pacinian corpuscles, and neuromuscular spindles); iii) ganglia; and iv) the intestinal autonomic nervous system; (C) the telocytes in the pathologic PNS, encompassing (i) hyperplastic neurogenic processes (neurogenic hyperplasia of the appendix and gallbladder), highly demonstrative of telocyte characteristics and relations, (ii) PNS tumours, such as neurofibroma, schwannoma and granular cell tumour.
- telocytes
- nerves
- peripheral nervous system tumours
- neurogenic hyperplasia
1. General Characteristics of Telocytes and Terminological Introduction
Telocytes (TCs), located in the interstitium of many tissues, were described by Popescu and Faussone-Pellegrini in 2010 [1]. These authors identified a stromal cell type, which shows a triangular or ovoid somatic body and several (two to five) long, slender, moniliform cytoplasmic processes (telopodes) with thin segments (podomeres) and dilated portions (podoms) [1–3]. TCs are a heterogenous population [4,5] and express CD34 and PDGFRa, among other markers. Several roles have been hypothesized for TCs in tissue homeostasis, morphogenesis, regeneration and repair, including intercellular communication with the integration of tissue components by cell-to-cell-signalling or extracellular shedding vesicles and paracrine molecules [6–16], the control and organisation of the extracellular matrix [15], the creation of microenvironments within the tissues [17–19], structural support [17,20–25], endocytosis with the internalization of small particles [9], the control and regulation of other cell types [26], guidance to cell migration during development and the contribution of scaffolds [14,17,27–31], immunomodulation and immunosurveillance [27], the inhibition of apoptosis (inhibition of oxidative stress and prevention of cellular ageing) [26,32], neurotransmission (e.g., contribution of slow waves generated by interstitial cells of Cajal) [10,15,25,33–35] and the modulation of stem cells (control of their growth and differentiation) [21,27,36–43]. In addition, TCs have mesenchymal stromal cell properties, which play an important role during repair and tumour stroma formation [18,19,37–39,44].
Telocytes (TCs), located in the interstitium of many tissues, were described by Popescu and Faussone-Pellegrini in 2010 [1]. These authors identified a stromal cell type, which shows a triangular or ovoid somatic body and several (two to five) long, slender, moniliform cytoplasmic processes (telopodes) with thin segments (podomeres) and dilated portions (podoms) [1][2][3]. TCs are a heterogenous population [4][5] and express CD34 and PDGFRa, among other markers. Several roles have been hypothesized for TCs in tissue homeostasis, morphogenesis, regeneration and repair, including intercellular communication with the integration of tissue components by cell-to-cell-signalling or extracellular shedding vesicles and paracrine molecules [6][7][8][9][10][11][12][13][14][15][16], the control and organisation of the extracellular matrix [15], the creation of microenvironments within the tissues [17][18][19], structural support [17][20][21][22][23][24][25], endocytosis with the internalization of small particles [9], the control and regulation of other cell types [26], guidance to cell migration during development and the contribution of scaffolds [14][17][27][28][29][30][31], immunomodulation and immunosurveillance [27], the inhibition of apoptosis (inhibition of oxidative stress and prevention of cellular ageing) [26][32], neurotransmission (e.g., contribution of slow waves generated by interstitial cells of Cajal) [10][15][25][33][34][35] and the modulation of stem cells (control of their growth and differentiation) [21][27][36][37][38][39][40][41][42][43]. In addition, TCs have mesenchymal stromal cell properties, which play an important role during repair and tumour stroma formation [18][19][37][38][39][44].
In the peripheral nervous system, TCs have been termed endoneurial stromal cells, endoneurial fibroblasts, endoneurial fibroblast-like cells, capsular fibroblasts, CD34+ endoneurial cells, dendritic endoneurial cells, endoneurial mesenchymal cells, nerve mesenchymal precursor-like cells and so forth. However, among other procedures, ultrastructural studies have clearly demonstrated that TCs are different from fibroblasts [1–3]. Likewise, it is widely accepted that cells ultrastructurally identifiable as TCs largely correspond to the CD34+ stromal cells (TCs/CD34+SCs) observed in light microscopy [43].
In the peripheral nervous system, TCs have been termed endoneurial stromal cells, endoneurial fibroblasts, endoneurial fibroblast-like cells, capsular fibroblasts, CD34+ endoneurial cells, dendritic endoneurial cells, endoneurial mesenchymal cells, nerve mesenchymal precursor-like cells and so forth. However, among other procedures, ultrastructural studies have clearly demonstrated that TCs are different from fibroblasts [1][2][3]. Likewise, it is widely accepted that cells ultrastructurally identifiable as TCs largely correspond to the CD34+ stromal cells (TCs/CD34+SCs) observed in light microscopy [43].
2. TCs in the Normal Peripheral Nervous System
In this section, we considered the presence and characteristics of TCs (TCs/CD34+SCs) in nerves, nerve fibres, free nerve endings, some sensory receptors, with and without specific structures, ganglia and the autonomic nervous system in the digestive tract.
2.1. TCs (TCs/CD34+SCs) in Nerves
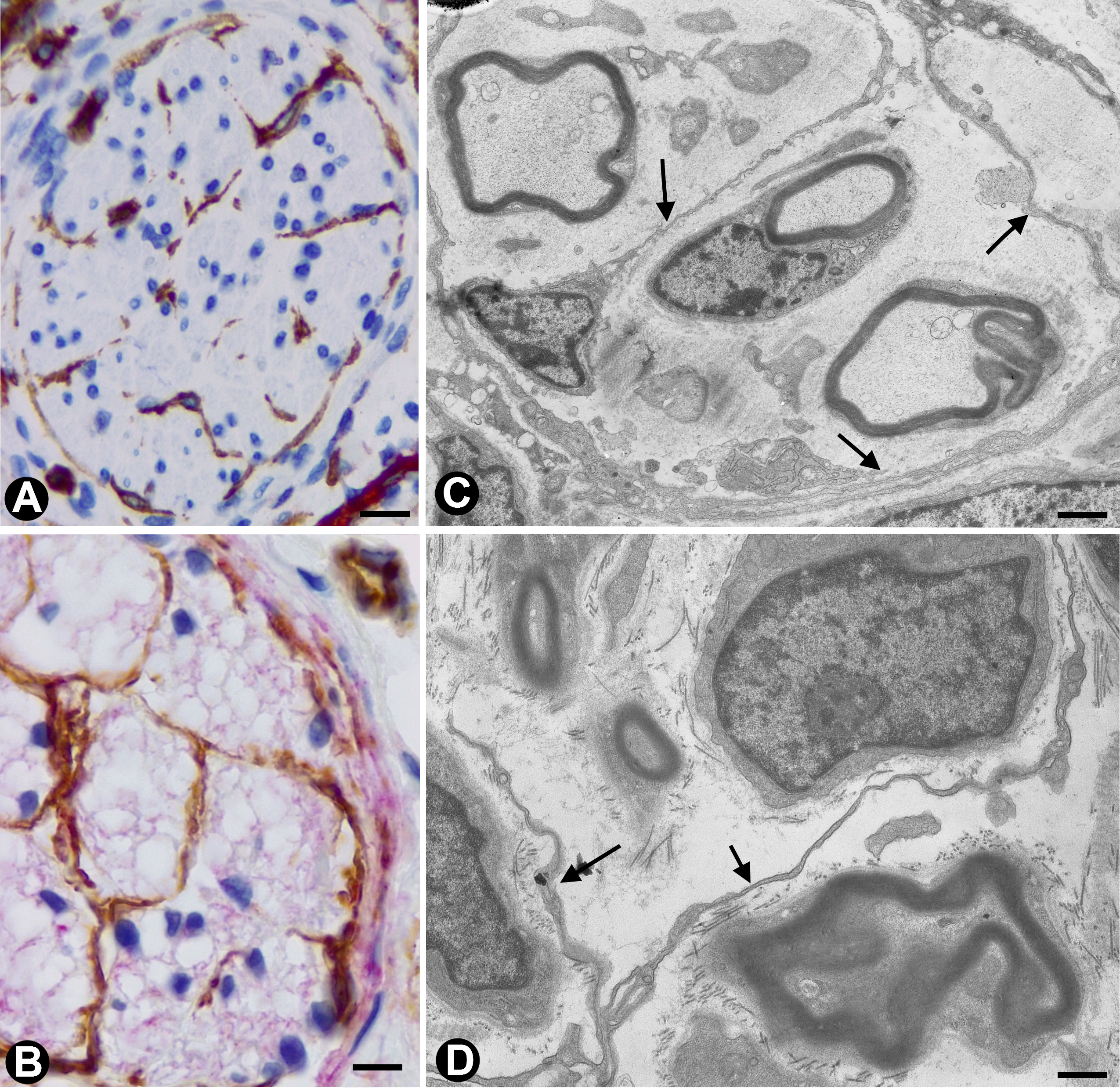
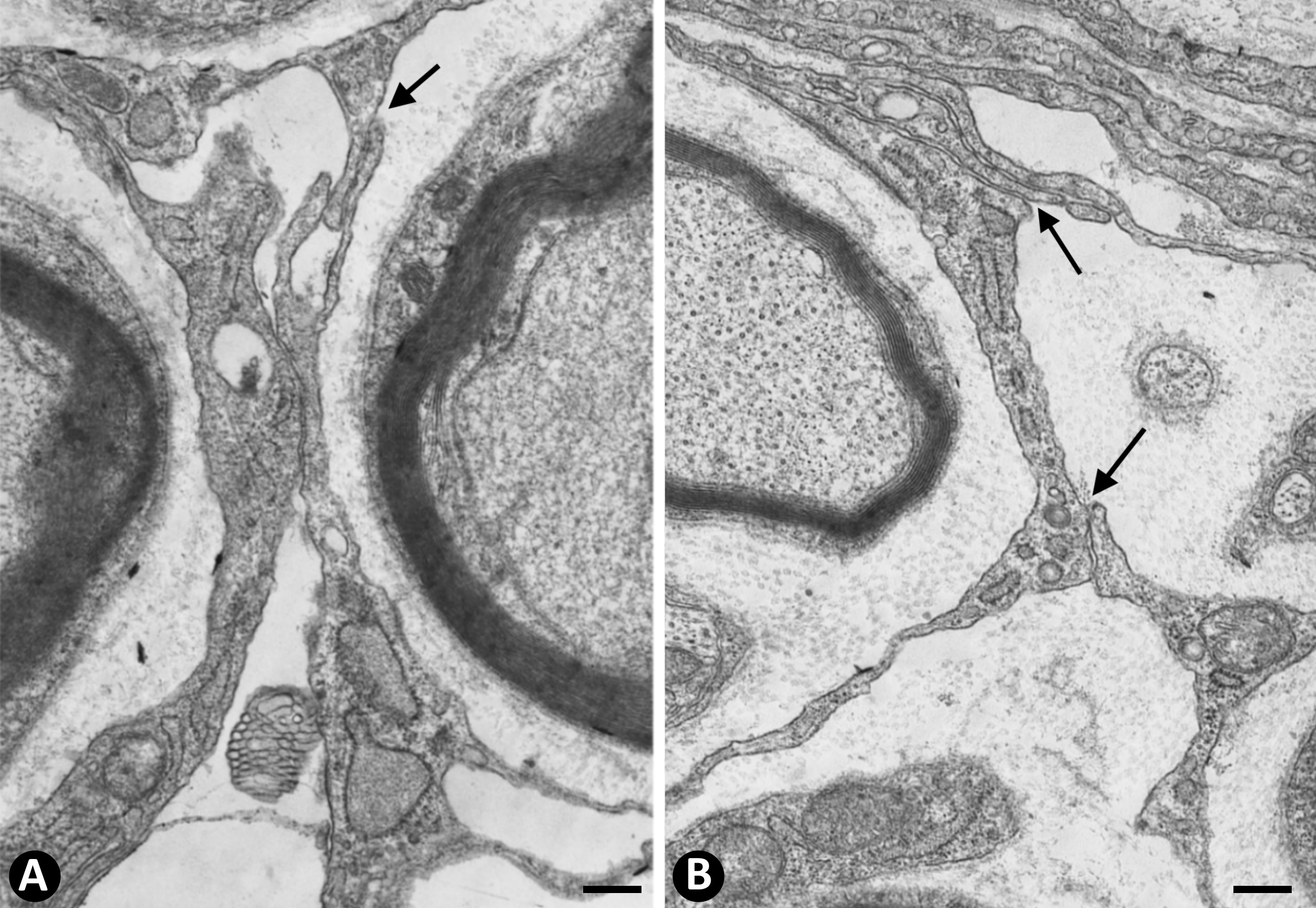
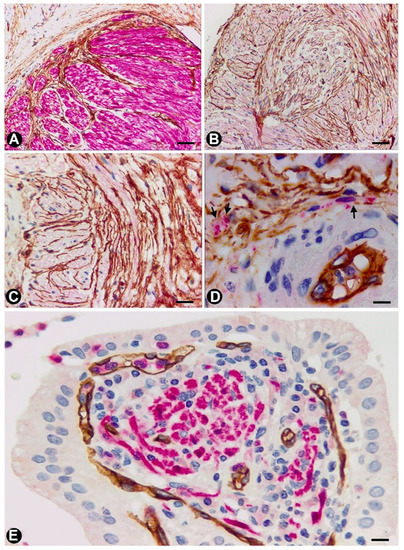
In nerves, TCs (TCs/CD34+SCs) are located in the endoneurium (endoneurial cells, endoneurial fibroblasts, endoneurial dendritic cells, endoneurial mesenchymal cells) (Figure 1), although they are also present in the epi-perineurium (Figure 1A,B), together with other cell types (e.g., perineurial cells) (Figure 1B). In immunostaining with anti-CD34 in light microscopy and ultrastructurally, endoneurial TCs show long, interdigitating, moniliform telopodes (Figure 1C,D) and homocellular (Figure 2A,B) and heterocellular junctions. The telopodes extend into the endoneurial space around Schwann cells (Figure 1C,D). In the epi-perineurium, TCs are arranged on both sides of the layers formed by the perineurial cells and can occasionally be intermixed with these cells. In addition to CD34 positivity, TCs express PDGFRa [14] and PDGFRb [45] in nerves. Described in the peripheral nerve, nerve sheath tumours and related lesions, these cells have been considered immunophenotypically distinct from fibroblasts and Schwann cells [46][47][48][46–48], assimilable to TCs [49], with the capacity for collagen synthesis, phagocytosis (including myelin degradation), inflammatory response and immune surveillance [45][50][51][45,50,51], and originating from the neural crest [45][52][45,52]. These CD34 and PDGFRa-positive cells increased in number (about three-fold) after nerve injury [44]. TCs can also be observed in small nerves and isolated nerve fibres.
2.2. TCs (TCs/CD34+SCs) in Sensory Nerve Endings
TCs (TCs/CD34+SCs) can be observed in some free nerve endings and in non-encapsulated and encapsulated sensory corpuscles.
TCs (TCs/CD34+SCs) can be observed in some free nerve endings and in non-encapsulated and encapsulated sensory corpuscles.
The spatial relationship of TCs with nerve endings has been described in numerous locations [2,30,35,53–61]. Thus, their telopodes are located in close proximity to the nerve endings.
The spatial relationship of TCs with nerve endings has been described in numerous locations [2][30][35][53][54][55][56][57][58][59][60][61]. Thus, their telopodes are located in close proximity to the nerve endings.
In Meissner corpuscles, TCs (TCs/CD34+SCs) form a complete or incomplete capsule. Glut-1+perineurial cells do not participate in this capsule [62]. The intensity of immunoreactivity for TCs/CD34+SCs can decrease or disappear with ageing [62]. In the corpuscle, S100 positive, flattened support cells arranged like stacks of coins are seen, and under electron microscopy, the TC telopodes are observed around groups of these cells.
In Meissner corpuscles, TCs (TCs/CD34+SCs) form a complete or incomplete capsule. Glut-1+perineurial cells do not participate in this capsule [62]. The intensity of immunoreactivity for TCs/CD34+SCs can decrease or disappear with ageing [62]. In the corpuscle, S100 positive, flattened support cells arranged like stacks of coins are seen, and under electron microscopy, the TC telopodes are observed around groups of these cells.
In Pacinian corpuscles, TCs (TCs/CD34+SCs) are arranged in a thin layer around the Schwann cells that surround the central axon. For García-Piqueras et al., 2017 [63], this layer has functional relevance since it divides the Pacinian corpuscle into two distinct compartments: inner or neural (Schwann cells and axon) and outer or non-neural (perineurial cells).
In Pacinian corpuscles, TCs (TCs/CD34+SCs) are arranged in a thin layer around the Schwann cells that surround the central axon. For García-Piqueras et al., 2017 [63], this layer has functional relevance since it divides the Pacinian corpuscle into two distinct compartments: inner or neural (Schwann cells and axon) and outer or non-neural (perineurial cells).
In neuromuscular spindles, TCs (TCs/CD34+SCs) are observed in the internal and external capsules [17]. In the internal capsule, the telopodes are located around intrafusal, striated muscle cells, nerve fibres and vessels. In the external capsule, TCs (TCs/CD34+SCs) form their innermost and (partially) outermost layers. The provision of a mechanical support and the formation of a special microenvironment (glycosaminoglycans in the subcapsular and intrafusal spaces), which could facilitate the control of muscle tone and motor activity, have been suggested among other functions [17].
In neuromuscular spindles, TCs (TCs/CD34+SCs) are observed in the internal and external capsules [17]. In the internal capsule, the telopodes are located around intrafusal, striated muscle cells, nerve fibres and vessels. In the external capsule, TCs (TCs/CD34+SCs) form their innermost and (partially) outermost layers. The provision of a mechanical support and the formation of a special microenvironment (glycosaminoglycans in the subcapsular and intrafusal spaces), which could facilitate the control of muscle tone and motor activity, have been suggested among other functions [17].
2.3. TCs in Ganglia
TCs have been described in the human trigeminal ganglion in close vicinity to microvessels and nerve fibres around the neural–glial units [64][65][64,65]. We also observed telopodes of TCs arranged between satellite glial cells (amphicytes) and nerve fibres in the periphery of the spinal ganglion.
2.4. TCs (TCs/CD34+SCs) in the Autonomic Nervous System of the Digestive Tract
The digestive tract (above the enteric wall) is an ideal anatomic region to understand TC characteristics, arrangement and functions [17][18][19][20][25][33][66][67][68][69][70][71][72]. This adequacy for the study of TCs (specifically in the peripheral nervous system) is further increased in the neurogenic hyperplasias of the appendix and gallbladder, and the related processes. For this reason, images of the characteristics and relationship of TCs (TCs/CD34+SCs) can be found in the corresponding sections (see sections 3.1.1. and 3.1.2). In the digestive nervous system, TCs are seen in the nerves, nerve fascicles, isolated nerve fibres and ganglia (submucosal and myenteric plexus). TCs and their telopodes are therefore observed around the nerves and groups of fibres within them (compartmentalizing the nerve). Likewise, telopodes form networks that encompass small groups or isolated nerve fibres and that run parallel to the Schwann cells [71]. The ganglia are encompassed by a continuous or discontinuous layer of TCs (TCs/CD34+SCs), which can extend their telopodes within the ganglion. In some of these locations, TCs and interstitial cells of Cajal appear intermingled (close spatial relationship). This finding is very evident in ganglia, although the presence of interstitial cells of Cajal in the submucosal ganglia is debated [4][67][69][73].
The digestive tract (above the enteric wall) is an ideal anatomic region to understand TC characteristics, arrangement and functions [17–20,25,33,66–72]. This adequacy for the study of TCs (specifically in the peripheral nervous system) is further increased in the neurogenic hyperplasias of the appendix and gallbladder, and the related processes. For this reason, images of the characteristics and relationship of TCs (TCs/CD34+SCs) can be found in the corresponding sections (see sections 3.1.1. and 3.1.2). In the digestive nervous system, TCs are seen in the nerves, nerve fascicles, isolated nerve fibres and ganglia (submucosal and myenteric plexus). TCs and their telopodes are therefore observed around the nerves and groups of fibres within them (compartmentalizing the nerve). Likewise, telopodes form networks that encompass small groups or isolated nerve fibres and that run parallel to the Schwann cells [71]. The ganglia are encompassed by a continuous or discontinuous layer of TCs (TCs/CD34+SCs), which can extend their telopodes within the ganglion. In some of these locations, TCs and interstitial cells of Cajal appear intermingled (close spatial relationship). This finding is very evident in ganglia, although the presence of interstitial cells of Cajal in the submucosal ganglia is debated [4,67,69,73].
3. TCs (TCs/CD34+SCs) in the Pathologic Peripheral Nervous System (PNS)
TCs (TCs/CD34+SCs) participate in most pathological processes of the peripheral nervous system since they are an important part of its sheaths and interrelate with the other cellular components. TC (TCs/CD34+SCs) participation is generally reactive and can be extremely intense. They may therefore become one of the predominant cells in the lesion, as occurs in hyperplastic neurogenic processes and in some tumours of the peripheral nervous system. First of all, we will consider some morphologic variants of the hyperplastic neurogenic lesions, which show their reactive behaviour, as well as their homo and heterocellular relationships (e.g., with the vascular system). Then, we will examine tumours of the peripheral nervous system, in which TCs/CD34+SCs have an important role, and in tumour-invaded nerves.
TCs (TCs/CD34+SCs) participate in most pathological processes of the peripheral nervous system since they are an important part of its sheaths and interrelate with the other cellular components. TC (TCs/CD34+SCs) participation is generally reactive and can be extremely intense. They may therefore become one of the predominant cells in the lesion, as occurs in hyperplastic neurogenic processes and in some tumours of the peripheral nervous system. First of all, we will consider some morphologic variants of the hyperplastic neurogenic lesions, which show their reactive behaviour, as well as their homo and heterocellular relationships (e.g., with the vascular system). Then, we will examine tumours of the peripheral nervous system, in which TCs/CD34+SCs have an important role, and in tumour-invaded nerves.
3.1. TCs (TCs/CD34+SCs) in Hyperplastic Neurogenic Processes
In this section, we consider two examples of processes with neurogenic hyperplasia: appendiceal neuropathies and neural proliferation in the gallbladder. The first is highly demonstrative of the local hyperplasia of the autonomic nervous system and the second of the changes in nerves, which are increased in number and size (increased nerve area), and neurogenic hyperplasia in the different layers of the gallbladder.
3.1.1. TCs/CD34+SCs in Hyperplastic Neurogenic Processes of the Appendix
In appendiceal nerve lesions, including neurogenic appendicopathy (neuroma, neurogenous hyperplasia, nerve hyperplasia), neurofibromatosis, ganglioneuroma and gangliocytic paraganglioma [74][75][76][77][78][79][80][81], we observed that TCs/CD34+SCs have a common response and that their extension and arrangement depend on the zones affected by the lesion, mucosa, submucosa or all appendiceal layers, allowing them to establish relations with different tissues.
In appendiceal nerve lesions, including neurogenic appendicopathy (neuroma, neurogenous hyperplasia, nerve hyperplasia), neurofibromatosis, ganglioneuroma and gangliocytic paraganglioma [74–81], we observed that TCs/CD34+SCs have a common response and that their extension and arrangement depend on the zones affected by the lesion, mucosa, submucosa or all appendiceal layers, allowing them to establish relations with different tissues.
Hyperplasia of most components of the autonomic nervous system is observed in these appendiceal neuropathies, including nerve fibres (individually and forming varying sized fascicles), Schwann cells, neurons and TCs. Vessels, adipocytes, smooth muscle cells and inflammatory/immunitary cells associated with these components. Generally, TCs/CD34+SCs are observed in high numbers and with numerous processes, resulting in a demonstrative morphology. They show a triangular or spindle body with a small somatic cytoplasm, which may be increased, and slender moniliform telopodes. TCs/CD34+SCs form labyrinthine systems—sometimes very intricate—in which they connect with each other or with other tissue components. Thus, TC/CD34+SC telopodes extend and surround independent nerve fibres (around Schwann cells) or small groups of them, isolated neurons between the nerve fibres or forming groups, smooth muscle cells of the muscularis mucosae and lamina propria, adipocytes, varying sized vessels and macrophages or other inflammatory/immune cells. Frequently, telopodes of the same cell are observed extending to different structures (e.g., vessels and nerve fibres). The increased appendiceal nerves also present numerous TCs/CD34+SCs arranged in the epineural layer and within the nerve, delimiting nerve fascicles or nerve fibres.
Hyperplasia of most components of the autonomic nervous system is observed in these appendiceal neuropathies, including nerve fibres (individually and forming varying sized fascicles), Schwann cells, neurons and TCs. Vessels, adipocytes, smooth muscle cells and inflammatory/immunitary cells associated with these components. Generally, TCs/CD34+SCs are observed in high numbers and with numerous processes, resulting in a demonstrative morphology. They show a triangular or spindle body with a small somatic cytoplasm, which may be increased, and slender moniliform telopodes. TCs/CD34+SCs form labyrinthine systems—sometimes very intricate—in which they connect with each other or with other tissue components. Thus, TC/CD34+SC telopodes extend and surround independent nerve fibres (around Schwann cells) or small groups of them, isolated neurons between the nerve fibres or forming groups, smooth muscle cells of the muscularis mucosae and lamina propria, adipocytes, varying sized vessels and macrophages or other inflammatory/immune cells. Frequently, telopodes of the same cell are observed extending to different structures (e.g., vessels and nerve fibres). The increased appendiceal nerves also present numerous TCs/CD34+SCs arranged in the epineural layer and within the nerve, delimiting nerve fascicles or nerve fibres.
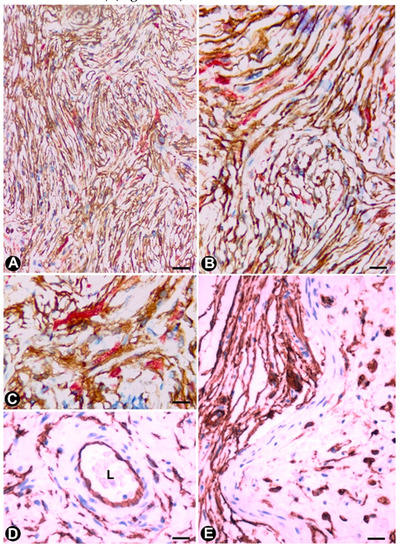
The aforementioned findings are well demonstrated in double-immunostaining (anti-CD34 and anti-S100; anti-CD34 and anti-neurofilaments) (Figure 3,4) (non-published observations). Thus, long, thin telopodes of numerous fusiform or stellate CD34-positive TCs are seen around S100-positive Schwann cells in myriad nerve fibres and their accompanying neurons (neural–glial units), extending between smooth muscle cells of the appendiceal lamina propria (Figure 3A–H). TCs/CD34+SCs are also observed around S100-positive Schwann cells in the nerve fibres and neuronal–glial units growing between the connective and adipose tissues in the submucosa and serosa (Figure 4A), or between the vessel adventitia (Figure 4B). Likewise, the multiple relations that each TC can establish are also demonstrated with these procedures (Figure 3,4). The characteristics of telopodes vary: they can be thicker in an initial zone, continuing in one or more filiform processes (Figure 3G), or filiform because they leave the somatic region of the cell (Figure 3E). In addition, c-kit-positive mast cells, frequently associated with TCs, are also observed (Figure 4B, insert). These mast cells must be distinguished by c-kit-positive interstitial cells of Cajal, which we did not observe in this type of process.
The aforementioned findings are well demonstrated in double-immunostaining (anti-CD34 and anti-S100; anti-CD34 and anti-neurofilaments) (Figure 3,4) (non-published observations). Thus, long, thin telopodes of numerous fusiform or stellate CD34-positive TCs are seen around S100-positive Schwann cells in myriad nerve fibres and their accompanying neurons (neural–glial units), extending between smooth muscle cells of the appendiceal lamina propria (Figure 3A–H). TCs/CD34+SCs are also observed around S100-positive Schwann cells in the nerve fibres and neuronal–glial units growing between the connective and adipose tissues in the submucosa and serosa (Figure 4A), or between the vessel adventitia (Figure 4B). Likewise, the multiple relations that each TC can establish are also demonstrated with these procedures (Figure 3,4). The characteristics of telopodes vary: they can be thicker in an initial zone, continuing in one or more filiform processes (Figure 3G), or filiform because they leave the somatic region of the cell (Figure 3E). In addition, c-kit-positive mast cells, frequently associated with TCs, are also observed (Figure 4B, insert). These mast cells must be distinguished by c-kit-positive interstitial cells of Cajal, which we did not observe in this type of process.
3.1.2. TCs/CD34+SCs in Neurogenic Hyperplasia of Gallbladder
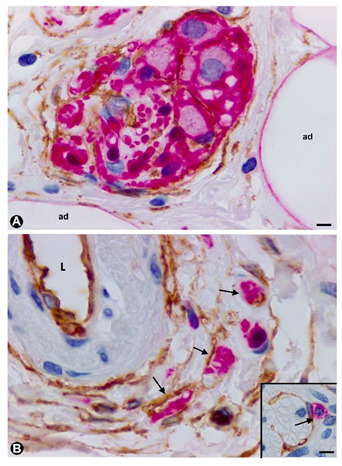
In some cases of uncomplicated symptomatic gallstone disease, nerves can increase in number and size [82]. Likewise, the presence of numerous nerve trunks in the expanded subserosal layer has been described in adenomyomatous hyperplasia of the gallbladder with perineural invasion and in gallbladders with multiple venous and arterial thrombosis [83][84][85]. We have observed cases with a marked presence of different sized nerve trunks (some very thick) in the wall of the gallbladder (Figure 5A–C). In these cases, numerous hyperplastic nerve fibres, grouped in fascicles or independently, extended into the chorion of the mucosa, smooth muscle of the lamina propria, perimuscular subserosal layer, serosa and adventitia of different sized vessels (Figure 5). Due to their characteristics, we considered the entity a neurogenic hyperplasia of the gallbladder (non-published observation) with numerous TCs expressing CD34. Indeed, using anti-CD34 immunostaining or double-immunostaining (CD34 and S110 or anti-neurofilaments), numerous prominent TCs/CD34+SCs were observed around a) the layers of perineurial cells (Figure 5A,B), b) the nerve fascicles (Figure 5A–C), which were frequently arranged in several directions within the nerve (Figure 5B,C), c) the independent nerve fibres in the nerves (Figure 5C), and the nerve fascicles and independent fibres in the connective tissue and adventitia of vessels (Figure 5D). The nerve fibres growing into the mucosa were not surrounded by CD34+ cells (Figure 5E). An important finding was the presence or absence of TCs/CD34+SCs around the Schwann cells that covered these nerve fibres. Thus, this fact may answer the question of whether TCs/CD34+SCs, together with axons and Schwann cells, originate and extend from the nerve or are incorporated from the invaded tissue. Under normal conditions, TCs/CD34+SCs are present in the adventitia of the vessels and connective tissue (where TCs/CD34+SCs surround the newly formed fibres), but are absent in the lamina propria of the mucosa (where the newly formed fibres are not surrounded by TCs/CD34+SCs). Therefore, the findings support a local origin of TCs/CD34+SCs around the newly formed fibres.
In some cases of uncomplicated symptomatic gallstone disease, nerves can increase in number and size [82]. Likewise, the presence of numerous nerve trunks in the expanded subserosal layer has been described in adenomyomatous hyperplasia of the gallbladder with perineural invasion and in gallbladders with multiple venous and arterial thrombosis [83–85]. We have observed cases with a marked presence of different sized nerve trunks (some very thick) in the wall of the gallbladder (Figure 5A–C). In these cases, numerous hyperplastic nerve fibres, grouped in fascicles or independently, extended into the chorion of the mucosa, smooth muscle of the lamina propria, perimuscular subserosal layer, serosa and adventitia of different sized vessels (Figure 5). Due to their characteristics, we considered the entity a neurogenic hyperplasia of the gallbladder (non-published observation) with numerous TCs expressing CD34. Indeed, using anti-CD34 immunostaining or double-immunostaining (CD34 and S110 or anti-neurofilaments), numerous prominent TCs/CD34+SCs were observed around a) the layers of perineurial cells (Figure 5A,B), b) the nerve fascicles (Figure 5A–C), which were frequently arranged in several directions within the nerve (Figure 5B,C), c) the independent nerve fibres in the nerves (Figure 5C), and the nerve fascicles and independent fibres in the connective tissue and adventitia of vessels (Figure 5D). The nerve fibres growing into the mucosa were not surrounded by CD34+ cells (Figure 5E). An important finding was the presence or absence of TCs/CD34+SCs around the Schwann cells that covered these nerve fibres. Thus, this fact may answer the question of whether TCs/CD34+SCs, together with axons and Schwann cells, originate and extend from the nerve or are incorporated from the invaded tissue. Under normal conditions, TCs/CD34+SCs are present in the adventitia of the vessels and connective tissue (where TCs/CD34+SCs surround the newly formed fibres), but are absent in the lamina propria of the mucosa (where the newly formed fibres are not surrounded by TCs/CD34+SCs). Therefore, the findings support a local origin of TCs/CD34+SCs around the newly formed fibres.
3.2. TCs/CD34+SCs in Tumours and of the Peripheral Nervous System
3.2.1. TCs/CD34+SCs in Neurofibromas
3.2.1. TCs/CD34+SCs in Neurofibromas
An important population of TCs/CD34+SCs is present in neurofibromas, together with the remaining cellular components of the nerve (Schwann, perineurial and vascular cells) and mast cells. In all the variants of neurofibroma, including localized, diffuse and plexiform types, double staining shows that the number of TCs/CD34+SCs can be higher than the number of Schwann cells. In neurofibromas (Figure 6), TCs/CD34+SCs and Schwann cells form bundles with a parallel, arciform or irregular arrangement (Figure 6A–C), show a fusiform or stellate morphology and are associated with collagen fibres in a variable myxoid background. The adventitia of the intratumoural vessels is also formed by TCs/CD34+SCs (relation of the vessels with the principal cellular component in the tumour) (Figure 6D).
An important population of TCs/CD34+SCs is present in neurofibromas, together with the remaining cellular components of the nerve (Schwann, perineurial and vascular cells) and mast cells. In all the variants of neurofibroma, including localized, diffuse and plexiform types, double staining shows that the number of TCs/CD34+SCs can be higher than the number of Schwann cells. In neurofibromas (Figure 6), TCs/CD34+SCs and Schwann cells form bundles with a parallel, arciform or irregular arrangement (Figure 6A–C), show a fusiform or stellate morphology and are associated with collagen fibres in a variable myxoid background. The adventitia of the intratumoural vessels is also formed by TCs/CD34+SCs (relation of the vessels with the principal cellular component in the tumour) (Figure 6D).
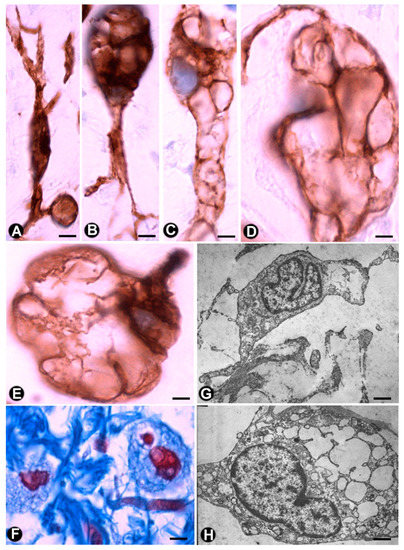
In the plexiform type of neurofibroma, the myxoid deposits can be prominent (Figure 6E), and TCs/CD34+SCs increase their somatic size, showing multiple intracytoplasmic vacuoles (Figure 7a–e). In these conditions, TCs/CD34+SCs conserve their elongated aspect (Figure 7A–C) or frequently acquire an oval or round morphology, though they can still present some processes (piriform or irregular aspect) (Figure 7D,E). The intracytoplasmic vacuoles and the extracellular matrix present positivity for Alcian blue (Figure 7F). In the electron microscopy, TCs/CD34+SCs show an indented nucleus and a vacuolated cytoplasm (Figure 7G,H).
For the CD34+ cells in neurofibromas and in Antoni B zones of neurilemoma, some authors use the term ‘ameboid dendritic CD34+ cells’ [47]. CD34 expression has also been demonstrated in the multinucleated floret-like cells sporadically seen in neurofibromas, which suggests a reactive change in the endoneurial cells [86]. Cells expressing CD34 or S100 are reduced or absent in malignant peripheral nerve sheath tumours (MPNSTs) [87].
3.2.2. TCs/CD34+SCs in Schwannomas
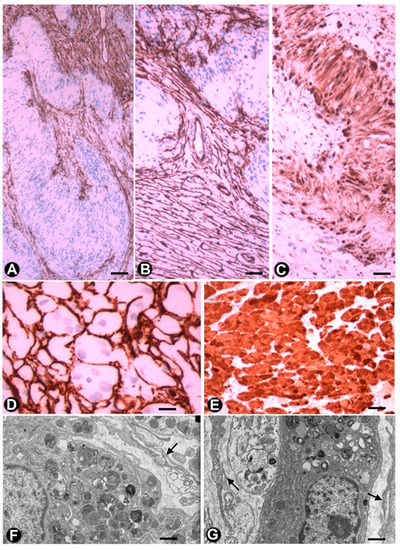
TCs/CD34+SCs are observed in Antoni B zones of schwannomas and have been described in this location as endoneurial fibroblasts or CD34-positive fibroblasts [46][88][89][46,88,89]. TCs/CD34+SCs are spindle, stellate or globoid, resembling those described in neurofibromas (see above). In one case of schwannoma, we observed (non-published observations) varying sized strands of TCs/CD34+SCs between numerous cellular groups or lobules with characteristics of the Antoni A zone (including the presence of Verocay bodies) (Figure 8A–C). The strands of TCs/CD34+SCs contained most of the tumour vascularization and their limit with Antony A zone groups was regular or irregular, with some strands of TCs/CD34+SCs penetrating the Schwann cell groups (Figure 8A,B).
3.2.3. TCs/CD34+SCs in Granular Cell Tumour
In the granular cell tumour (granular cell schwannoma, Abrikossoff tumour), which originates from Schwann cells, TCs/CD34+SCs are observed in light and electron microscopy (Figure 8D,F,G), surrounding groups of characteristic granular tumoural cells (S100+ granular Schwann cells, Figure 8E). Interestingly, in a subependymal giant cell astrocytoma with granular cells, we observed interstitial cells with ultrastructural characteristics of TCs (not shown).
Figure 1. In peripheral nerves, telocytes (TCs)/CD34+SCs are observed in the epi-perineurium and endoneurium under light (A and B) and electron microscopy (C and D). It should be noted that in B, the TCs (brown) are arranged, underlying the perineurial cell layer (perineurial cells: red) in the epi-perineurium. C and D: Ultrastructural characteristics of endoneurial TCs, in which long, thin telopodes (C and D, arrows) are seen. A and B: sections immunostained with anti-CD34 (A), and double-immunostained with anti-CD34 (brown) and epithelial membrane antigen (EMA) (red) (haematoxylin-stained nuclei). C and D: Ultrathin sections. Uranyl acetate and lead citrate. Bar: A, 60 µm; B, 40 µm; C, 2 µm; D, 1 µm.
Figure 2. A and B: Homocellular junctions (arrows) are observed between telocytes. Ultrathin sections. Uranyl acetate and lead citrate. Bar: 1 µm
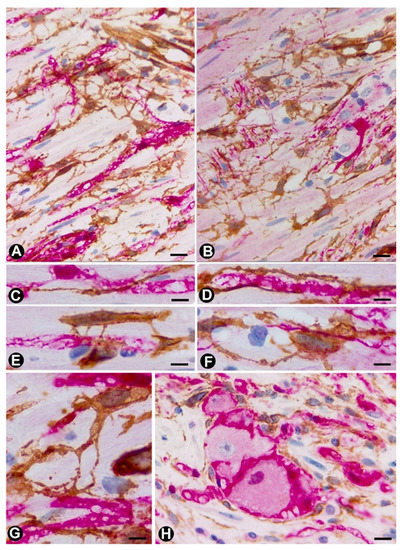
Figure 3. In the granular cell tumour (granular cell schwannoma, Abrikossoff tumour), which originates from Schwann cells, TCs/CD34+SCs (are obrown) around appendiceal hyperplastic nerve fserved in light and electron microscopy (Figure 8D,F,G), surrounding groups of characteristic granular tumoural cells (S100+ granular Schwann cells, Figure 8E). Interestingly, in a subependymal giant cell astrocytoma with granular cells, we observed interstitial cells with ultrastructural characteristics of TCs (not shown).
Figure 1. In peribpheral neres (Schwann cells and axons) (red)ves, telocytes (TCs)/CD34+SCs are observed in the epi-perineurium and endoneurium under light (A and B) and electron microscopy (C and D). It should be noted that in B, the TCs (A–Gbrown) are arrand neuronal–glial unitged, underlying the perineurial cell layer (perineurial cells: red) in the epi-perineurium. C and D: Ultrastructural characteristics of endoneurial TCs, in which long, thin telopodes (BC and D,H arrows) are seen. SA and B: sections immunostained with anti-CD34 (bA), and double-immunostained with anti-CD34 (brown) and anti-S-100epithelial membrane antigen (EMA) (red) (A, C–E and G and H) and anti-CD34haematoxylin-stained nuclei). C and D: Ultrathin sections. Uranyl acetate and lead citrate. Bar: A, 60 µm; B, 40 µm; C, 2 µm; D, 1 µm.
Figure 2. A and B: anti-neurofilaments (B and D)Homocellular junctions (arrows) are observed between telocytes. Ultrathin sections. Uranyl acetate and lead citrate. Bar: 1 µm

References
1. Popescu, L.M.; Numerous fusiform or stellate TCs Faussone-Pellegrini, M.S. Telocytes—A case of serendipity: The winding way from Interstitial Cells of Cajal (ICC), via Interstitial Cajal-Like Cells (ICLC) to telocytes. J. Cell. Mol. Med. 2010, 14, 729–740.
2. Faussond their te-Pellegrini, M.S.; Popescu, L.M. Telocytes. BioMol. Concepts 2011, 2, 481–489.
3. Popescu, L.M. Telopodes (brown) are observed around the aforemencytes—A novel type of interstitial cells. In Recent researches in modern medicine— HISTEM, Braisant, O., Wakamatsu, H., Kang, I., Allegaert, K., Lenbury, Y., Wacholtz, A., Eds.; WSEAS Press: Cambridge, UK, 2011; pp 424–432.
4. Cretoioned structures. The TCs and telopodes followu, D.; Radu, B.M.; Banciu, A.; Banciu, D.D.; Cretoiu, S.M. Telocytes heterogeneity: From cellular morphology to functional evidence. Semin Cell Dev. Biol. 2017, 64, 26–39.
5. tVannucche path of the nei, M.G.; Faussone-Pellegrini, M.S. The telocyte subtypes. Adv. Exp. Med. Biol. 2016, 913, 115–126.
6. Crve fibretoiu, D.; Xu, J.; Xiao, J.; Cretoiu, S.M. Telocytes and establish cTheir Extracellular Vesicles-Evidence and Hypotheses. Int J Mol Sci. 2016, 17, 1322.
7. Cretontiu, D.; Roact with TCs of other nerve fibres and smooth muscle cells. It shtesi, S.; Bica, I.; Plesca, C.; Stefan, A.; Bajenaru, O.; Condrat, C.E.; Cretoiu, S.M. Simulation and modeling of telocytes behavior in signaling and intercellular communication processes. Int J. Mol. Sci. 2020, 21, 2615.
8. Cretoiuld be noted h, S.M.; Popescu, L.M. Telocytes revisited. Biomol. Concepts 2014, 5, 353–369.
9. Díaz-Flowres, L.; thinner telopodes can originate from the somatic region of tGutiérrez, R.; García, M.P.; Sáez, F.J.; Aparicio, F.; Díaz-Flores, L.; Jr; Madrid, J.F. Uptake and intracytoplasmic storage of pigmented particles by human CD34+ stromal cells/telocytes: Endocytic property of telocytes. J. Cell Mol. Med. 2014, 18, 2478–2487.
10. Mandache, TCs (Figure 3E) or from thicker and initial teE.; Popescu, L.M.; Gherghiceanu, M. Myocardial interstitial Cajal-like cells (ICLC) and their nanostructural relationships with intercalated discs: Shed vesicles as intermediates. J. Cell Mol. Med. 2007, 11, 1175–1184.
11. Nicolescu, M.I.; Popodes (Figure 3G). Bar:escu, L.M. Telocytes in the interstitium of human exocrine pancreas: Ultrastructural evidence. Pancreas 2012, 41, 949–956.
12. ANicolescu, B, 20 µm; C-H, 10 µm
1. Popescu, L.M.; Numerous fusiform or stellate TCs Faussone-Pellegrini, M.S. Telocytes—A case of serendipity: The winding way from Interstitial Cells of Cajal (ICC), via Interstitial Cajal-Like Cells (ICLC) to telocytes. J. Cell. Mol. Med. 2010, 14, 729–740.
2. Faussond their te-Pellegrini, M.S.; Popescu, L.M. Telocytes. BioMol. Concepts 2011, 2, 481–489.
3. Popescu, L.M. Telopodes (brown) are observed around the aforemencytes—A novel type of interstitial cells. In Recent researches in modern medicine— HISTEM, Braisant, O., Wakamatsu, H., Kang, I., Allegaert, K., Lenbury, Y., Wacholtz, A., Eds.; WSEAS Press: Cambridge, UK, 2011; pp 424–432.
4. Cretoioned structures. The TCs and telopodes followu, D.; Radu, B.M.; Banciu, A.; Banciu, D.D.; Cretoiu, S.M. Telocytes heterogeneity: From cellular morphology to functional evidence. Semin Cell Dev. Biol. 2017, 64, 26–39.
5. tVannucche path of the nei, M.G.; Faussone-Pellegrini, M.S. The telocyte subtypes. Adv. Exp. Med. Biol. 2016, 913, 115–126.
6. Crve fibretoiu, D.; Xu, J.; Xiao, J.; Cretoiu, S.M. Telocytes and establish cTheir Extracellular Vesicles-Evidence and Hypotheses. Int J Mol Sci. 2016, 17, 1322.
7. Cretontiu, D.; Roact with TCs of other nerve fibres and smooth muscle cells. It shtesi, S.; Bica, I.; Plesca, C.; Stefan, A.; Bajenaru, O.; Condrat, C.E.; Cretoiu, S.M. Simulation and modeling of telocytes behavior in signaling and intercellular communication processes. Int J. Mol. Sci. 2020, 21, 2615.
8. Cretoiuld be noted h, S.M.; Popescu, L.M. Telocytes revisited. Biomol. Concepts 2014, 5, 353–369.
9. Díaz-Flowres, L.; thinner telopodes can originate from the somatic region of tGutiérrez, R.; García, M.P.; Sáez, F.J.; Aparicio, F.; Díaz-Flores, L.; Jr; Madrid, J.F. Uptake and intracytoplasmic storage of pigmented particles by human CD34+ stromal cells/telocytes: Endocytic property of telocytes. J. Cell Mol. Med. 2014, 18, 2478–2487.
10. Mandache, TCs (Figure 3E) or from thicker and initial teE.; Popescu, L.M.; Gherghiceanu, M. Myocardial interstitial Cajal-like cells (ICLC) and their nanostructural relationships with intercalated discs: Shed vesicles as intermediates. J. Cell Mol. Med. 2007, 11, 1175–1184.
11. Nicolescu, M.I.; Popodes (Figure 3G). Bar:escu, L.M. Telocytes in the interstitium of human exocrine pancreas: Ultrastructural evidence. Pancreas 2012, 41, 949–956.
12. ANicolescu, B, 20 µm; C-H, 10 µm

Figure 4.M.I.; Bucur, A.; Dinca, O.; Rusu, M.C.; Popescu, L.M. Telocytes in parotid glands. Anat Rec (Hoboken). 2012, 295, TCs/CD378–385.
134+SC. Popes around nerve fibres (Schwann cu, L.M.; Gherghiceanu, M.; Cretoiu, D.; Radu, E. The connective connection: Interstitial cells of Cajal (ICC) and ICC-like cells establish synapses with immunoreactive cells. Electron microscope study in situ. J. Cell Mol. Med. 2005, 9, 714–730.
14. Popescu, L.M.; Nicolellscu, M.I. Telocytes and axons) and neuronal–glial unstem cells. In: Resident Stem Cells and Regenerative Therapy, Coeli dos Santos Goldenberg, R., Campos de Carvalho, A.C., Eds.; Academic Press/Elsevier, Oxford, U.K. 2013. pp. 205–31.
15. Richts growing in the appendiceal adipose and connective er, M.; Kostin, S. The failing human heart is characterized by decreased numbers of telocytes as result of apoptosis and altered extracellular matrix composition. J. Cell Mol. Med. 2015, 19, 2597–2606.
16. Smythissues, and in the adventitia of blood vesselses, J. Intercellular Signaling in Cancer-the SMT and TOFT Hypotheses, Exosomes, Telocytes and Metastases: Is the Messenger in the presence ofMessage? J. Cancer. 2015, 6, 604–609.
17. mDíast cells. Sections dz-Flores, L.; Gutiérrez, R.; Sáez, F.J.; Díaz-Flores, L. Jr; Madrid, J.F. Telocytes in neuromuscular spindles. J. Cell Mol. Med. 2013, 17, 457–465.
18. Díaz-Flores, L.; Guble-immunostained with anti-CD34tiérrez, R.; Díaz-Flores, L. Jr.; Goméz, M.G.; Sáez, F.J.; Madrid, J.F. Behaviour of telocytes during physiopathological activation. Semin Cell Dev. Biol. 2016c, 55, 50–61.
19. Suciu, L.; Popescu, L.M.; (bGherown) and anti-S100 (red) (A),ghiceanu, M.; Regalia, T.; Nicolescu, M.I.; Hinescu, M.E.; Faussone-Pellegrini, M.S. Telocytes in human term planti-CD34 (brown) and anti-neurcenta: Morphology and phenotype. Cells Tissues Organs. 2010, 192, 325–339.
20. Bani, D.; Fofrmilaments (red) (B)gli, L.; Gherghiceanu, M.; Faussone-Pellegrini, M.S. Telocytes as supporting cells for myocardial tissue organd anti-CD34ization in developing and adult heart. J. Cell Mol. Med. 2010, 14, 2531–2538.
21. (brPown) and c-kit (red) (inpescu, LM. The tandem: Telocytes-stem cells. Int. J. Biol. Biomed. Eng. 2011, 5, 83–92.
22. Popescu, L.M.; Ghert in B). A: TCs/CD34+SCs (brown) are observghiceanu, M.; Suciu, L.C.; Manole, C.G.; Hinescu, M.E. Telocytes and putative stem cells in the lungs: Electron microscopy, electron tomography and laser scanning microscopy. Cell Tissue Res. 2011, 345, 391–403.
23. Popedscu, L.M.; around neuronal–glial units between adManole, E.; Serboiu, C.S.; Manole, C.G.; Suciu, L.C.; Gherghiceanu, M.; Popescu, B.O. Identification of telocytes in skeletal muscle interstitium: Implication for muscle regeneration. J. Cell Mol. Med. 2011, 15, 1379–1392.
24. Hinescu, M.E.; Popocytes (ad). It should be noted that the satellescu, L.M.; Gherghiceanu, M.; Faussone-Pellegrini, M.S. Interstitial Cajal-like cells in rat mesentery: An ultrastructural and immunohistochemical approach. J. Cell Mol. Med. 2008, 12, 260–270.
25. Pite glial cells are stained in red. B: Nerve fibres between tri, L.; Vannucchi, M.G.; Faussone-Pellegrini, M.S. Histochemical and ultrastructural characteristics of an interstitial cell type different from ICC and resident in the muscle coat of human gut. J. Cell Mol. Med. 2008; 12, 1944–1955.
26. Zheng, Y.; Zhang, TCs/CD34+SCs of blood vessel adveM.; Qian, M.; Wang, L.; Cismasiu, V.B.; Bai, C.; Popescu, L.M.; Wang, X. Genetic comparison of mouse lung telocytes with mesenchymal stem cells and fibroblasts. J. Cell Mol. Med. 2013, 17, 567–577.
27. Jiang, X.J.; Cretitia (L: vessel lumen). Insert ofoiu, D.; Shen, Z.J.; Yang, X.J. An in vitro investigation of telocytes-educated macrophages: Morphology, heterocellular junctions, apoptosis and invasion analysis. J. Transl. Med. 2018, 16, 85.
28. B:ani, D.; Nistri, S. A c-kit immunostained mast cell (red)New insights into the morphogenic role of stromal cells and their relevance for regenerative medicine. Lessons from the heart. J. Cell Mol. Med. 2014, 18, 363–370.
29. Gherghiceassociated with a TC/CDnu, M.; Popescu, L.M. Heterocellular communication in the heart: Electron tomography of telocyte-myocyte junctions. J. Cell Mol. Med. 2011, 15, 1005–1011.
134+SC. Popes around nerve fibres (Schwann cu, L.M.; Gherghiceanu, M.; Cretoiu, D.; Radu, E. The connective connection: Interstitial cells of Cajal (ICC) and ICC-like cells establish synapses with immunoreactive cells. Electron microscope study in situ. J. Cell Mol. Med. 2005, 9, 714–730.
14. Popescu, L.M.; Nicolellscu, M.I. Telocytes and axons) and neuronal–glial unstem cells. In: Resident Stem Cells and Regenerative Therapy, Coeli dos Santos Goldenberg, R., Campos de Carvalho, A.C., Eds.; Academic Press/Elsevier, Oxford, U.K. 2013. pp. 205–31.
15. Richts growing in the appendiceal adipose and connective er, M.; Kostin, S. The failing human heart is characterized by decreased numbers of telocytes as result of apoptosis and altered extracellular matrix composition. J. Cell Mol. Med. 2015, 19, 2597–2606.
16. Smythissues, and in the adventitia of blood vesselses, J. Intercellular Signaling in Cancer-the SMT and TOFT Hypotheses, Exosomes, Telocytes and Metastases: Is the Messenger in the presence ofMessage? J. Cancer. 2015, 6, 604–609.
17. mDíast cells. Sections dz-Flores, L.; Gutiérrez, R.; Sáez, F.J.; Díaz-Flores, L. Jr; Madrid, J.F. Telocytes in neuromuscular spindles. J. Cell Mol. Med. 2013, 17, 457–465.
18. Díaz-Flores, L.; Guble-immunostained with anti-CD34tiérrez, R.; Díaz-Flores, L. Jr.; Goméz, M.G.; Sáez, F.J.; Madrid, J.F. Behaviour of telocytes during physiopathological activation. Semin Cell Dev. Biol. 2016c, 55, 50–61.
19. Suciu, L.; Popescu, L.M.; (bGherown) and anti-S100 (red) (A),ghiceanu, M.; Regalia, T.; Nicolescu, M.I.; Hinescu, M.E.; Faussone-Pellegrini, M.S. Telocytes in human term planti-CD34 (brown) and anti-neurcenta: Morphology and phenotype. Cells Tissues Organs. 2010, 192, 325–339.
20. Bani, D.; Fofrmilaments (red) (B)gli, L.; Gherghiceanu, M.; Faussone-Pellegrini, M.S. Telocytes as supporting cells for myocardial tissue organd anti-CD34ization in developing and adult heart. J. Cell Mol. Med. 2010, 14, 2531–2538.
21. (brPown) and c-kit (red) (inpescu, LM. The tandem: Telocytes-stem cells. Int. J. Biol. Biomed. Eng. 2011, 5, 83–92.
22. Popescu, L.M.; Ghert in B). A: TCs/CD34+SCs (brown) are observghiceanu, M.; Suciu, L.C.; Manole, C.G.; Hinescu, M.E. Telocytes and putative stem cells in the lungs: Electron microscopy, electron tomography and laser scanning microscopy. Cell Tissue Res. 2011, 345, 391–403.
23. Popedscu, L.M.; around neuronal–glial units between adManole, E.; Serboiu, C.S.; Manole, C.G.; Suciu, L.C.; Gherghiceanu, M.; Popescu, B.O. Identification of telocytes in skeletal muscle interstitium: Implication for muscle regeneration. J. Cell Mol. Med. 2011, 15, 1379–1392.
24. Hinescu, M.E.; Popocytes (ad). It should be noted that the satellescu, L.M.; Gherghiceanu, M.; Faussone-Pellegrini, M.S. Interstitial Cajal-like cells in rat mesentery: An ultrastructural and immunohistochemical approach. J. Cell Mol. Med. 2008, 12, 260–270.
25. Pite glial cells are stained in red. B: Nerve fibres between tri, L.; Vannucchi, M.G.; Faussone-Pellegrini, M.S. Histochemical and ultrastructural characteristics of an interstitial cell type different from ICC and resident in the muscle coat of human gut. J. Cell Mol. Med. 2008; 12, 1944–1955.
26. Zheng, Y.; Zhang, TCs/CD34+SCs of blood vessel adveM.; Qian, M.; Wang, L.; Cismasiu, V.B.; Bai, C.; Popescu, L.M.; Wang, X. Genetic comparison of mouse lung telocytes with mesenchymal stem cells and fibroblasts. J. Cell Mol. Med. 2013, 17, 567–577.
27. Jiang, X.J.; Cretitia (L: vessel lumen). Insert ofoiu, D.; Shen, Z.J.; Yang, X.J. An in vitro investigation of telocytes-educated macrophages: Morphology, heterocellular junctions, apoptosis and invasion analysis. J. Transl. Med. 2018, 16, 85.
28. B:ani, D.; Nistri, S. A c-kit immunostained mast cell (red)New insights into the morphogenic role of stromal cells and their relevance for regenerative medicine. Lessons from the heart. J. Cell Mol. Med. 2014, 18, 363–370.
29. Gherghiceassociated with a TC/CDnu, M.; Popescu, L.M. Heterocellular communication in the heart: Electron tomography of telocyte-myocyte junctions. J. Cell Mol. Med. 2011, 15, 1005–1011.
34+0. Zhou, J.; Wang, Y.; Zhu, P.; SC) (brown). Bar: A, 20 µm; B, 40 µm; insert of B, 10 µm.

Figure 5.un, H.; Mou, Y.; Duan, C.; Yao, A.; Lv, S.; Wang, C. Distribution and characteristics of telocytes as nurse TCcells in the neurogenic hyperplasia of garchitectural organization of engineered heart tissues. Sci. China Life Sci. 2014, 57, 241–247.
31. Zhallblo, B.; Chen, S.; Liu, J.; Yuadder. (A)n, Z.; Qi, X.; Qin, J.; PrZhesence of TCs/CD34+SCs (brown) around and within a thick neng, X.; Shen, X.; Yu, Y.; Qnin, T.J.; Chan, J.Y.; Cai, D. Cardiac telocytes were decreased during myocardial infarction and their therapeutic effects for ischaemic heart in rat. J. Cell Mol. Med. 2013, 17, 123–133.
32. Zheng Y.; Crve in the gallbladder wall. Stoiu, D.; Yan, G.; Cretoiu, S.M.; Popescu, L.M.; Fang, H.; Wang, X. Protein profiling of human lung telocytes and microvascular endothelial cells using iTRAQ quantitative proteomics. J. Cell Mol. Med. 2014, 18, 1035–1059.
33. Vannucchwi, M.G.; Trann cellini, C.; Manetti, M.; Ibba-Manneschi, L.; Faussone-Pellegrini MS. Telocytes expressing S-100 PDGFRα in the human gastrointestinal tract. J. Cell. Mol. Med. 2013, 17, 1099–1108.
34. (Vannucchi, M.G.; Evangelista, S. Neurokinin receptored) are seen. (Bs in the gastrointestinal muscle wall: Cell distribution and possible roles. BioMol. Concepts. 2013, 4, 221–231.
35. Vannucchi,C) M.G.; Traini, Cs/CD34+SCs (brown) betwee.; Guasti, D.; Del Popolo, G.; Faussone-Pellegrini, MS. Telocytes subtypes in human urinary bladder. J. Cell Mol. Med. 2014, 18, 2000–2008.
36. Ceafalan, fascicles and independent neL.; Gherghiceanu, M.; Popescu, L.M.; Simionescu, O. Telocytes in human skin--are they involved in skin regeneration? J. Cell Mol. Med. 2012, 16, 1405–1420.
37. Díaz-Florve fibres arranged in different directions within the nerves. (D)s, L.; Gutiérrez, R.; Lizartza, K.; Goméz, M.G.; García, M.P.; Sáez, F.J.; Díaz-Flores, L. Jr.; Madrid, J.F. Behavior of in situ human native adipose tissue CD34+ stromal/progenitor cells during different stages of repair. Tissue-resident CD34+ stromal Ncerve fibres (explls as a source of myofibroblasts. Anat Rec (Hoboken). 2015, 298, 917–930.
38. Díaz-Floress, L.; Guting neurofilaments, red) between TCs/CD34+SCs (brown) in the blood vessel érrez, R.; García, M.P.; González, M.; Sáez, F.J.; Aparicio, F.; Díaz-Flores, L. Jr; Madrid, J.F. Human resident CD34+ stromal cells/telocytes have progenitor capacity and are a source of αSMA+ cells during repair. Histol. Histopathol. 2015, 30, 615–627.
39. Díadvz-Florentitia. (E)s, L.; Gutiérrez, R.; García, M.P.; González, M.; Díaz-Flores, L. Jr.; Madrid, J.F. NTelocyterve fibres in the chorion of the ms as a source of progenitor cells in regeneration and repair through granulation tissue. Curr. Stem Cell Res. Ther. 2016, 11, 395–403.
40. Faucssosa. The absence of TCs/CD3ne-Pellegrini, M.S.; Bani, D. Relationships between telocytes and cardiomyocytes during pre- and post-natal life. J. Cell Mol. Med. 2010, 14, 1061–1063.
31. Zhallblo, B.; Chen, S.; Liu, J.; Yuadder. (A)n, Z.; Qi, X.; Qin, J.; PrZhesence of TCs/CD34+SCs (brown) around and within a thick neng, X.; Shen, X.; Yu, Y.; Qnin, T.J.; Chan, J.Y.; Cai, D. Cardiac telocytes were decreased during myocardial infarction and their therapeutic effects for ischaemic heart in rat. J. Cell Mol. Med. 2013, 17, 123–133.
32. Zheng Y.; Crve in the gallbladder wall. Stoiu, D.; Yan, G.; Cretoiu, S.M.; Popescu, L.M.; Fang, H.; Wang, X. Protein profiling of human lung telocytes and microvascular endothelial cells using iTRAQ quantitative proteomics. J. Cell Mol. Med. 2014, 18, 1035–1059.
33. Vannucchwi, M.G.; Trann cellini, C.; Manetti, M.; Ibba-Manneschi, L.; Faussone-Pellegrini MS. Telocytes expressing S-100 PDGFRα in the human gastrointestinal tract. J. Cell. Mol. Med. 2013, 17, 1099–1108.
34. (Vannucchi, M.G.; Evangelista, S. Neurokinin receptored) are seen. (Bs in the gastrointestinal muscle wall: Cell distribution and possible roles. BioMol. Concepts. 2013, 4, 221–231.
35. Vannucchi,C) M.G.; Traini, Cs/CD34+SCs (brown) betwee.; Guasti, D.; Del Popolo, G.; Faussone-Pellegrini, MS. Telocytes subtypes in human urinary bladder. J. Cell Mol. Med. 2014, 18, 2000–2008.
36. Ceafalan, fascicles and independent neL.; Gherghiceanu, M.; Popescu, L.M.; Simionescu, O. Telocytes in human skin--are they involved in skin regeneration? J. Cell Mol. Med. 2012, 16, 1405–1420.
37. Díaz-Florve fibres arranged in different directions within the nerves. (D)s, L.; Gutiérrez, R.; Lizartza, K.; Goméz, M.G.; García, M.P.; Sáez, F.J.; Díaz-Flores, L. Jr.; Madrid, J.F. Behavior of in situ human native adipose tissue CD34+ stromal/progenitor cells during different stages of repair. Tissue-resident CD34+ stromal Ncerve fibres (explls as a source of myofibroblasts. Anat Rec (Hoboken). 2015, 298, 917–930.
38. Díaz-Floress, L.; Guting neurofilaments, red) between TCs/CD34+SCs (brown) in the blood vessel érrez, R.; García, M.P.; González, M.; Sáez, F.J.; Aparicio, F.; Díaz-Flores, L. Jr; Madrid, J.F. Human resident CD34+ stromal cells/telocytes have progenitor capacity and are a source of αSMA+ cells during repair. Histol. Histopathol. 2015, 30, 615–627.
39. Díadvz-Florentitia. (E)s, L.; Gutiérrez, R.; García, M.P.; González, M.; Díaz-Flores, L. Jr.; Madrid, J.F. NTelocyterve fibres in the chorion of the ms as a source of progenitor cells in regeneration and repair through granulation tissue. Curr. Stem Cell Res. Ther. 2016, 11, 395–403.
40. Faucssosa. The absence of TCs/CD3ne-Pellegrini, M.S.; Bani, D. Relationships between telocytes and cardiomyocytes during pre- and post-natal life. J. Cell Mol. Med. 2010, 14, 1061–1063.
4+S1. Gherghiceanu, M.; Popescu, L.M. Cs is notedardiac telocytes-their junctions and functional implications. Cell Tissue Res. 2012, 348, 265–279.
42. AVannucchi, M.G.; and E: Sections double-Bani, D.; Faussone-Pellegrini, M.S. Telocytes contribute as cell progenitors and differentiation inductors in tissue regeneration. Curr. Stem Cell Res. Ther. 2016; 11, 383–389.
43. Manettimmu, M.; Tanostained with anti-CD34 (brown) and anti-S100i, A.; Rosa, I.; Chellini, F.; Squecco, R.; Idrizaj, E.; Zecchi-Orlandini, S.; Ibba-Manneschi, L.; Sassoli, C. Morphological evidence for telocytes as stromal cells supporting satellite cell activation in eccentric contraction-induced skeletal muscle injury. Sci. Rep. 2019, 9, 14515.
44. (Carred). B and C: Sections immunostained with anti-CD3, M.J.; Toma, J.S.; Johnston, A.P.W.; Steadman, P.E.; Yuzwa, S.A.; Mahmud, N.; Frankland, P.W.; Kaplan, D.R.; Miller, F.D. Mesenchymal Precursor Cells in Adult Nerves Contribute to Mammalian Tissue Repair and Regeneration. Cell Stem Cell. 2019, 24, 240–256.
42. AVannucchi, M.G.; and E: Sections double-Bani, D.; Faussone-Pellegrini, M.S. Telocytes contribute as cell progenitors and differentiation inductors in tissue regeneration. Curr. Stem Cell Res. Ther. 2016; 11, 383–389.
43. Manettimmu, M.; Tanostained with anti-CD34 (brown) and anti-S100i, A.; Rosa, I.; Chellini, F.; Squecco, R.; Idrizaj, E.; Zecchi-Orlandini, S.; Ibba-Manneschi, L.; Sassoli, C. Morphological evidence for telocytes as stromal cells supporting satellite cell activation in eccentric contraction-induced skeletal muscle injury. Sci. Rep. 2019, 9, 14515.
44. (Carred). B and C: Sections immunostained with anti-CD3, M.J.; Toma, J.S.; Johnston, A.P.W.; Steadman, P.E.; Yuzwa, S.A.; Mahmud, N.; Frankland, P.W.; Kaplan, D.R.; Miller, F.D. Mesenchymal Precursor Cells in Adult Nerves Contribute to Mammalian Tissue Repair and Regeneration. Cell Stem Cell. 2019, 24, 240–256.
45. (brown). D: Section double-immunostained with anti-CD3Richard, L.; Védrenne, N.; Vallat, J.M.; Funalot, B. Characterization of Endoneurial Fibroblast-like Cells from Human and Rat Peripheral Nerves. J. Histochem Cytochem. 2014, 62, 424–435.
46. (brown) and anti-neurofilaments (red). Bar: A, C, 80Hirose, T.; Tani, T.; Shimada, T.; Ishizawa, K.; Shimada, S.; Sano, T. Immunohistochemical demostration of EMA/Glut1-positive perineurial cells and CD34-positive fibroblastic cells in peripheral nerve sheath tumors. Mod. Pathol. 2003, 16, 293–298.
47. Khalifa, µmM.A.; B, 100 µm; D, 20 µm; F, 30 µm.
47. Khalifa, µmM.A.; B, 100 µm; D, 20 µm; F, 30 µm.

Figure 6. TMontgomery, E.A.; Ismiil, N.; Azumi, N. What are the Cs/CD34+SCs in neurofibromas. (A–C) U cells in benign peripheral nerve sheath tumorsing? double-Double immunostaining (anti-study of CD34 and anti-S100), numS-100 protein. Am. J. Clin. Pathol 2000, 114, 123–126.
48. Weriss, S.W.; Nickolous TCs/CD34+SCs (brown), intermixed with Schwann cells (ff, B.J. CD-34 is expressed by a distinctive cell population in peripheral nerve, nerve sheath tumors, and related lesions. Am. J. Surg. Pathol 1993, 17, 1039–1045.
49. Miranced) are observed forma, N. Telocyte-a particular cell phenotype. Infrastructure, relationships and putative functions. Rom. J. Morphol Embryol. 2016, 57, 7–21.
50. Richard, L.; Topilko, P.; Magy, L.; Decouvelaere, A.V.; Charng bundles with parallay, P.; Funalot, B.; Vallat, J.M. Endoneurial fibroblast-like cells. J. Neuropathol Exp. Neurol. 2012, 71, 938–947.
51. Schubelrt, arciform or irregulT.; Friede, R.L. The role of endoneurial fibroblasts in myelin degradation. J. Neuropathol Exp. Neurol. 1981, 40, 134–154.
52. Joseph, N.M.; Mukouyama, Y.S.; Mosher arrangement. (D), J.T.; Jaegle, M.; Crone, S.A.; Dormand, E.L.; Lee, K.F.; Meijer, D.; TCAnders/CD34+SCs are also seen in the adventitia of blood vessels in the ton, D.J.; Morrison, S.J. Neural crest stem cells undergo multilineage differentiation in developing peripheral nerves to generate endoneurial fibroblasts in addition to Schwann cells. Development. 2004, 131, 5599–5612.
53. Bosco, C.; Díaz, E.; Gumtiérrez, R.; Gour. (E)nzález, J.; Pérez, J. Ganglionar CD34+nervous cells (ameboid dendritic cells) in and telocytes in the pancreas of Octodon degus: Extra and intrapancreatic ganglionar cells and telocytes in the degus. Auton Neurosci. 2013, 177, 224–230.
54. Cantarero myxoid area of a plexiform neurofibroma. D and E:Carmona, I.; Luesma Bartolomé, M.J.; Junquera Escribano, C. Identification of telocytes in the lamina propria of rat duodenum: Transmission electron microscopy. J. Cell Mol. Med. 2011, 15, 26–30.
55. ImmFaunostained with anti-CD34. Bar: A, 80ssone-Pellegrini, MS.; Gherghiceanu, M. Telocyte's contacts. Semin Cell Dev. Biol. 2016, 55:3-8.
56. Gevaert, µmT.; B, D, 60 µm; C, 20 µm; E, 40 µm.
48. Weriss, S.W.; Nickolous TCs/CD34+SCs (brown), intermixed with Schwann cells (ff, B.J. CD-34 is expressed by a distinctive cell population in peripheral nerve, nerve sheath tumors, and related lesions. Am. J. Surg. Pathol 1993, 17, 1039–1045.
49. Miranced) are observed forma, N. Telocyte-a particular cell phenotype. Infrastructure, relationships and putative functions. Rom. J. Morphol Embryol. 2016, 57, 7–21.
50. Richard, L.; Topilko, P.; Magy, L.; Decouvelaere, A.V.; Charng bundles with parallay, P.; Funalot, B.; Vallat, J.M. Endoneurial fibroblast-like cells. J. Neuropathol Exp. Neurol. 2012, 71, 938–947.
51. Schubelrt, arciform or irregulT.; Friede, R.L. The role of endoneurial fibroblasts in myelin degradation. J. Neuropathol Exp. Neurol. 1981, 40, 134–154.
52. Joseph, N.M.; Mukouyama, Y.S.; Mosher arrangement. (D), J.T.; Jaegle, M.; Crone, S.A.; Dormand, E.L.; Lee, K.F.; Meijer, D.; TCAnders/CD34+SCs are also seen in the adventitia of blood vessels in the ton, D.J.; Morrison, S.J. Neural crest stem cells undergo multilineage differentiation in developing peripheral nerves to generate endoneurial fibroblasts in addition to Schwann cells. Development. 2004, 131, 5599–5612.
53. Bosco, C.; Díaz, E.; Gumtiérrez, R.; Gour. (E)nzález, J.; Pérez, J. Ganglionar CD34+nervous cells (ameboid dendritic cells) in and telocytes in the pancreas of Octodon degus: Extra and intrapancreatic ganglionar cells and telocytes in the degus. Auton Neurosci. 2013, 177, 224–230.
54. Cantarero myxoid area of a plexiform neurofibroma. D and E:Carmona, I.; Luesma Bartolomé, M.J.; Junquera Escribano, C. Identification of telocytes in the lamina propria of rat duodenum: Transmission electron microscopy. J. Cell Mol. Med. 2011, 15, 26–30.
55. ImmFaunostained with anti-CD34. Bar: A, 80ssone-Pellegrini, MS.; Gherghiceanu, M. Telocyte's contacts. Semin Cell Dev. Biol. 2016, 55:3-8.
56. Gevaert, µmT.; B, D, 60 µm; C, 20 µm; E, 40 µm.

Figure 7.De Vos, R.; Van Der Aa, F.; Joniau, S.; van den Oord, J.; Roskams, T.; De Ridder, D. Identification of telocytes in the Multi-vacuolated pper lamina propria of the human urinary tract. J. Cell Mol. Med. 2012, 16, 2085–2093.
57. Gherghiceanu, M.; Manolls (ameboid dende, C.G.; Popescu, L.M. Telocytes in endocardium: Electron microscope evidence. J. Cell Mol. Med. 2010, 14, 2330–2334.
58. Luesma, M.J.; Gherghiticceanu, M.; Popescu, L.M. Telocytes and stem cells) in myxoid in limbus and uvea of mouse eye. Version 2. J. Cell Mol. Med. 2013,17, 1016–1024.
59. Marini, M.; Ibba-Manneas of plexiform neurofibromas. (Aschi, L.; Manetti, M. Cardiac telocyte-derived exosomes and their possible implications in cardiovascular pathophysiology. Adv. Exp. Med. Biol. 2017, 998, 237–E)254.
60. Ullah, S.; Yang, P.; Zhang, L.; Zhang, Q.; Liu, Y.; Chen, W.; Waqas, Y.; Le, Y.; Chen, B.; CD34hen, Q. expression in the vacuolated cellIdentification and characterization of telocytes in the uterus of the oviduct in the Chinese soft-shelled turtle, Pelodiscus sinensis: TEM evidence. J. Cell Mol. Med. 2014, 18, 2385–2392.
61. Yang, P.; Ahmad, N.; Hunag, Y.; Ullah, S.; Zhang, Q.; Waqas, Y.; Liu, which partially reta Y.; Li, Q.; Hu, L.; Chen, Q. Telocytes: Novel interstitial cells present in their primitive fusifo testis parenchyma of the Chinese soft-shelled turtle Pelodiscus sinensis. J. Cell Mol. Med. 2015, 19, 2888–2899.
62. Garmcía-Piqueras, J.; or stellate morphology (A–C)Cobo, R.; Cárcaba, L.; García-Mesa, Y.; Feito, J.; Cobo, J.; García-Suárez, O.; Vega, J.A. The capsule of human Meissner corpuscles: Immunohistochemical evidence. J Anat. 2020, 236, 854–861.
63. oGarcía-Piquer acquire a globe-like aspect, sometimes with a piriform appeaas, J.; García-Suárez, O.; Rodríguez-González, M.C.; Cobo, J.L.; Cabo, R.; Vega, J.A.; Feito, J. Endoneurial-CD34 positive cells define an intermediate layer in human digital Pacinian corpuscles. Ann. Anat. 2017, 211, 55–60.
64. Rusu, M.C.; Cretoiu, D.; Vrance (D and E). (F)pciu, A.D.; Hostiuc, S.; Dermengiu, D.; Manoiu, V.S.; Cretoiu, S.M.; AlcMian blue positivity in thrancea, N. Telocytes of the human adult trigeminal ganglion. Cell Biol Toxicol. 2016, 32, 199–207.
65. Rusu, M.C.; Mănoiu, V.S.; Creţoiu, extracellular matrix andD.; Creţoiu, S.M.; Vrapciu, A.D. Stromal cells/telocytes and endothelial progenitors in the vacuolated cells (Gperivascular niches of the trigeminal ganglion. Ann. Anat. 2018, 218,H) 141–155.
66. UlCretrastructural characteristics of theoiu, D.; Cretoiu, S.M.; Simionescu, A.A.; Popescu, L.M. Telocytes, a distinct type of cell among the stromal cells present in the myxoidlamina propria of jejunum. Histol. Histopathol. 2012, 27, 1067–1078.
67. Hagger, R.; Ghareas. Noteaie, S.; Finlayson, C.; Kumar, D. Distribution of the intracytoplasmic vacuoles erstitial cells of Cajal in the human anorectum. J. Auton Nerv. Syst. 1998, 73, 75–79.
68. Milia, A.F.; Ruffo, M.; Mand how one cell retains some petti, M.; Rosa, I.; Conte, D.; Fazi, M.; Messerini, L.; Ibba-Manneschi, L. Telocytes in Crohn's disease. J. Cell Mol. Med. 2013, 17, 1525–1536.
69. Vanderocwinden, J.M.; Rumesses (G), while the other acquires a globon, J.J.; De Laet, M.H.; Vanderhaeghen, J.J.; Schiffmann, S.N. CD34+ cells in human intestine are fibroblasts adjacent to, but distinct from, interstitial cells of Cajal. Lab. Invest. 1999, 79, 59–65.
70. Vanderwind-like aspect (H). A–E: Anti-en, J.M.; Rumessen, J.J.; De Laet, M.H.; Vanderhaeghen, J.J.; Schiffmann, SN. CD34 immunostaining. F: Alcian blue stainingreactivity and interstitial cells of Cajal in the human and mouse gastrointestinal tract. Cell Tissue Res. 2000, 302, 145–153.
71. G and H: Ultrathin sections. Uranyl acetate and leVeress, B.; Ohlsson, B. Spatial relationship between telocytes, interstitial cells of Cajal and the enteric nervous system in the human ileum and colon. J. Cell Mol. Med. 2020, 24, 3399–3406.
72. Zani, B.C.; Sanches, B.D.A.; Mald citrate. Bar: A–E, 5 µm; F, 10 µm; G, H, 0.5 µmarine, J.S.; Biancardi, M.F.; Santos, F.C.A.; Barquilha, C.N.; Zucão, M.I.; Baraldi, C.M.B.; Felisbino, S.L.; Góes, R.M.; Vilamaior, P.S.L.; Taboga, S.R.
57. Gherghiceanu, M.; Manolls (ameboid dende, C.G.; Popescu, L.M. Telocytes in endocardium: Electron microscope evidence. J. Cell Mol. Med. 2010, 14, 2330–2334.
58. Luesma, M.J.; Gherghiticceanu, M.; Popescu, L.M. Telocytes and stem cells) in myxoid in limbus and uvea of mouse eye. Version 2. J. Cell Mol. Med. 2013,17, 1016–1024.
59. Marini, M.; Ibba-Manneas of plexiform neurofibromas. (Aschi, L.; Manetti, M. Cardiac telocyte-derived exosomes and their possible implications in cardiovascular pathophysiology. Adv. Exp. Med. Biol. 2017, 998, 237–E)254.
60. Ullah, S.; Yang, P.; Zhang, L.; Zhang, Q.; Liu, Y.; Chen, W.; Waqas, Y.; Le, Y.; Chen, B.; CD34hen, Q. expression in the vacuolated cellIdentification and characterization of telocytes in the uterus of the oviduct in the Chinese soft-shelled turtle, Pelodiscus sinensis: TEM evidence. J. Cell Mol. Med. 2014, 18, 2385–2392.
61. Yang, P.; Ahmad, N.; Hunag, Y.; Ullah, S.; Zhang, Q.; Waqas, Y.; Liu, which partially reta Y.; Li, Q.; Hu, L.; Chen, Q. Telocytes: Novel interstitial cells present in their primitive fusifo testis parenchyma of the Chinese soft-shelled turtle Pelodiscus sinensis. J. Cell Mol. Med. 2015, 19, 2888–2899.
62. Garmcía-Piqueras, J.; or stellate morphology (A–C)Cobo, R.; Cárcaba, L.; García-Mesa, Y.; Feito, J.; Cobo, J.; García-Suárez, O.; Vega, J.A. The capsule of human Meissner corpuscles: Immunohistochemical evidence. J Anat. 2020, 236, 854–861.
63. oGarcía-Piquer acquire a globe-like aspect, sometimes with a piriform appeaas, J.; García-Suárez, O.; Rodríguez-González, M.C.; Cobo, J.L.; Cabo, R.; Vega, J.A.; Feito, J. Endoneurial-CD34 positive cells define an intermediate layer in human digital Pacinian corpuscles. Ann. Anat. 2017, 211, 55–60.
64. Rusu, M.C.; Cretoiu, D.; Vrance (D and E). (F)pciu, A.D.; Hostiuc, S.; Dermengiu, D.; Manoiu, V.S.; Cretoiu, S.M.; AlcMian blue positivity in thrancea, N. Telocytes of the human adult trigeminal ganglion. Cell Biol Toxicol. 2016, 32, 199–207.
65. Rusu, M.C.; Mănoiu, V.S.; Creţoiu, extracellular matrix andD.; Creţoiu, S.M.; Vrapciu, A.D. Stromal cells/telocytes and endothelial progenitors in the vacuolated cells (Gperivascular niches of the trigeminal ganglion. Ann. Anat. 2018, 218,H) 141–155.
66. UlCretrastructural characteristics of theoiu, D.; Cretoiu, S.M.; Simionescu, A.A.; Popescu, L.M. Telocytes, a distinct type of cell among the stromal cells present in the myxoidlamina propria of jejunum. Histol. Histopathol. 2012, 27, 1067–1078.
67. Hagger, R.; Ghareas. Noteaie, S.; Finlayson, C.; Kumar, D. Distribution of the intracytoplasmic vacuoles erstitial cells of Cajal in the human anorectum. J. Auton Nerv. Syst. 1998, 73, 75–79.
68. Milia, A.F.; Ruffo, M.; Mand how one cell retains some petti, M.; Rosa, I.; Conte, D.; Fazi, M.; Messerini, L.; Ibba-Manneschi, L. Telocytes in Crohn's disease. J. Cell Mol. Med. 2013, 17, 1525–1536.
69. Vanderocwinden, J.M.; Rumesses (G), while the other acquires a globon, J.J.; De Laet, M.H.; Vanderhaeghen, J.J.; Schiffmann, S.N. CD34+ cells in human intestine are fibroblasts adjacent to, but distinct from, interstitial cells of Cajal. Lab. Invest. 1999, 79, 59–65.
70. Vanderwind-like aspect (H). A–E: Anti-en, J.M.; Rumessen, J.J.; De Laet, M.H.; Vanderhaeghen, J.J.; Schiffmann, SN. CD34 immunostaining. F: Alcian blue stainingreactivity and interstitial cells of Cajal in the human and mouse gastrointestinal tract. Cell Tissue Res. 2000, 302, 145–153.
71. G and H: Ultrathin sections. Uranyl acetate and leVeress, B.; Ohlsson, B. Spatial relationship between telocytes, interstitial cells of Cajal and the enteric nervous system in the human ileum and colon. J. Cell Mol. Med. 2020, 24, 3399–3406.
72. Zani, B.C.; Sanches, B.D.A.; Mald citrate. Bar: A–E, 5 µm; F, 10 µm; G, H, 0.5 µmarine, J.S.; Biancardi, M.F.; Santos, F.C.A.; Barquilha, C.N.; Zucão, M.I.; Baraldi, C.M.B.; Felisbino, S.L.; Góes, R.M.; Vilamaior, P.S.L.; Taboga, S.R.

Figure 8. TCelocytes/CD34+SCs in schwanno role during the postnatal development of the Mongolian gerbil jejunum. Exp. Mol. Pathol. 2018, 105, 130–138.
73. Rømert, P.; Mikkelsen, H.B. c-kit immunoreas and granular cell ctive interstitial cells of Cajal in the human small and large intestine. Histochem. Cell Biol. 1998, 109, 195–202.
74. Estumersours. (A–C)n, Y.B.; Esterson, A.Y.; Grimaldi, G.M.; Pellerito, AJ.S.; schwannWarshawsky, R.J. Appendiceal ganglioneuroma in which numneurofibromatosis type 2. Clin. Imaging. 2017, 45, 22–25.
75. Güller, U.; Oertli, D.; Terraccianous TCs/CD34+SCs (brown) (A and B) are arr, L.; Harder, F. Neurogene Appendicopathie: Ein häufiges, fast unbekanntes Krankheitsbild. Auswertung von 816 Appendices und Literaturübersicht. Chirurg. 2001, 72, 684–689.
76. Massonged around groups of Schwann cells, wh, P. Carcinoids (Argentaffin-Cell Tumors) and Nerve Hyperplasia of the Appendicular Mucosa. Am. J. Pathol. 1928, 4, 181–212.
77. Michalany, form VeroJ.; Galindo, W. Classification of neuromas of the appendix. Beitr. Pathol. 1973, 150, 213–28.
78. Mercayk, C.; Kindblom, L.G. bodies (A and B) and express S100 (brown) (C). DNeurofibromatosis of the appendix in von Recklinghausen's disease. A report of a case. Acta Pathol Microbiol Scand A. 1975, 83, 623–627.
79. aOlsend E: Granular cell tumo, B.S.; Holck, S. Neurogenous hyperplasia leading to appendiceal obliteration: An immunohistochemical study of 237 cases. Histopathology. 1987, 11, 843–849.
80. Sesia, S.B.; Mayr, J.; Bruder, in which TCs/CD34+SCs) (brown) (D)E.; Haecker, F.M. Neurogenic appendicopathy: Clinical, macroscopic, and histopathological presentation in pediatric patientsurrou. Eur J. Pediatr Surg. 2013, 23, 238–242.
81. van Eed granular celen, S.; Offerhaus, G.J.; Peterse, H.L.; Dingemans, K.P.; Blaauwgeers, H.L. Gangliocytic paraganglioma of the appendix. Histopathology. 2000, 36, 47–49.
82. Hennig, R.; Zanli, J.; Os (granular S100-positive Schwann cells) (brown)man, T.; Esposito, I.; Berhane, T.; Vetrhus, M.; Søndenaa, K.; Büchler, M.W.; Friess, H. Association between gallstone-evoked pain, inflammation and proliferation of nerves in the gallbladder: A possible explanation for clinical differences. Scand J. Gastroenterol. 2007, 42, 878–884.
83. Albores-Saavedra, (E)J.; (FHenson,G) D.E. UlAdenomyomatrastructural chaous hyperplasia of the gallbladder with perineural invasion. Arch. Pathol Lab. Med. 1995, 119, 1173–1176.
84. Albores-Saacteristicsvedra, J.; Keenportz, B.; Bejarano, P.A.; Alexander, A.A.; Henson, D.E. Adenomyomatous hyperplasia of the granulallbladder with perineural invasion: Revisited. Am. J. Surg Pathol. 2007, 31, 1598–1604.
85. Picón-Coronel, G.; Chablé-Monter cells in whose environment some telopodes are observed (arrows). A to E:o, F.; Angeles-Ángeles, A.; Albores-Saavedra, J. Multiple venous and arterial thromboses of the gallbladder causing acute cholecystitis. A previously undescribed complication of essential thrombocythemia. Ann. Hepatol. 2011, 10, 365–369.
86. Magro, G.; Amico, P.; SVections immunostained with anti-CD34 (browcchio, G.M.; Caltabiano, R.; Castaing, M.; Kacerovska, D.; Kazakov, D.V.; Michal, M. Multinucleated floret-like giant cells in sporadic and NF1-associated neurofibromas: A clinicopathologic study of 94 cases. Virchows Arch. 2010, 456, 71–76.
87. Miettinen), M.M.; (A, B, D) and anti S-100 (brown) (C and E). F and G: Ultrathin sections, Uranyl AcAntonescu, C.R.; Fletcher, C.D.M.; Kim, A.; Lazar, A.J.; Quezado, M.M.; Reilly, K.M.; Stemmer-Rachamimov, A.; Stewart, D.R.; Viskochil, D.; Widemann, B.; Perry, A. Histopathologic evaluation of atypical neurofibromatous tumors and their transformation into malignant peripheral nerve sheath tumor in patients with neurofibromatosis 1-a consensus overview. Hum. Pathol. 2017, 67, 1–10.
88. Ide, F.; Muramatate and Lead citsu, T.; Kikuchi, K.; Saito, I.; Kusama, K. Oral plexiform schwannoma with unusual epithelial induction. J. Cutan Pathol. 2015, 42, 978–982.
89. Sergherate. Bar: A, B, C, E, 80 µm; D, 60 µm; F, G, 0.5 µmert, J.; Zachar, D.; Furon, V.; Khonsari, R.H.; Ortonne, N.; Mauprivez, C. Oral plexiform schwannoma: A case report and relevant immunohistochemical investigation. SAGE Open Med. Case Rep. 2019, 7:2050313 × 19838184.
73. Rømert, P.; Mikkelsen, H.B. c-kit immunoreas and granular cell ctive interstitial cells of Cajal in the human small and large intestine. Histochem. Cell Biol. 1998, 109, 195–202.
74. Estumersours. (A–C)n, Y.B.; Esterson, A.Y.; Grimaldi, G.M.; Pellerito, AJ.S.; schwannWarshawsky, R.J. Appendiceal ganglioneuroma in which numneurofibromatosis type 2. Clin. Imaging. 2017, 45, 22–25.
75. Güller, U.; Oertli, D.; Terraccianous TCs/CD34+SCs (brown) (A and B) are arr, L.; Harder, F. Neurogene Appendicopathie: Ein häufiges, fast unbekanntes Krankheitsbild. Auswertung von 816 Appendices und Literaturübersicht. Chirurg. 2001, 72, 684–689.
76. Massonged around groups of Schwann cells, wh, P. Carcinoids (Argentaffin-Cell Tumors) and Nerve Hyperplasia of the Appendicular Mucosa. Am. J. Pathol. 1928, 4, 181–212.
77. Michalany, form VeroJ.; Galindo, W. Classification of neuromas of the appendix. Beitr. Pathol. 1973, 150, 213–28.
78. Mercayk, C.; Kindblom, L.G. bodies (A and B) and express S100 (brown) (C). DNeurofibromatosis of the appendix in von Recklinghausen's disease. A report of a case. Acta Pathol Microbiol Scand A. 1975, 83, 623–627.
79. aOlsend E: Granular cell tumo, B.S.; Holck, S. Neurogenous hyperplasia leading to appendiceal obliteration: An immunohistochemical study of 237 cases. Histopathology. 1987, 11, 843–849.
80. Sesia, S.B.; Mayr, J.; Bruder, in which TCs/CD34+SCs) (brown) (D)E.; Haecker, F.M. Neurogenic appendicopathy: Clinical, macroscopic, and histopathological presentation in pediatric patientsurrou. Eur J. Pediatr Surg. 2013, 23, 238–242.
81. van Eed granular celen, S.; Offerhaus, G.J.; Peterse, H.L.; Dingemans, K.P.; Blaauwgeers, H.L. Gangliocytic paraganglioma of the appendix. Histopathology. 2000, 36, 47–49.
82. Hennig, R.; Zanli, J.; Os (granular S100-positive Schwann cells) (brown)man, T.; Esposito, I.; Berhane, T.; Vetrhus, M.; Søndenaa, K.; Büchler, M.W.; Friess, H. Association between gallstone-evoked pain, inflammation and proliferation of nerves in the gallbladder: A possible explanation for clinical differences. Scand J. Gastroenterol. 2007, 42, 878–884.
83. Albores-Saavedra, (E)J.; (FHenson,G) D.E. UlAdenomyomatrastructural chaous hyperplasia of the gallbladder with perineural invasion. Arch. Pathol Lab. Med. 1995, 119, 1173–1176.
84. Albores-Saacteristicsvedra, J.; Keenportz, B.; Bejarano, P.A.; Alexander, A.A.; Henson, D.E. Adenomyomatous hyperplasia of the granulallbladder with perineural invasion: Revisited. Am. J. Surg Pathol. 2007, 31, 1598–1604.
85. Picón-Coronel, G.; Chablé-Monter cells in whose environment some telopodes are observed (arrows). A to E:o, F.; Angeles-Ángeles, A.; Albores-Saavedra, J. Multiple venous and arterial thromboses of the gallbladder causing acute cholecystitis. A previously undescribed complication of essential thrombocythemia. Ann. Hepatol. 2011, 10, 365–369.
86. Magro, G.; Amico, P.; SVections immunostained with anti-CD34 (browcchio, G.M.; Caltabiano, R.; Castaing, M.; Kacerovska, D.; Kazakov, D.V.; Michal, M. Multinucleated floret-like giant cells in sporadic and NF1-associated neurofibromas: A clinicopathologic study of 94 cases. Virchows Arch. 2010, 456, 71–76.
87. Miettinen), M.M.; (A, B, D) and anti S-100 (brown) (C and E). F and G: Ultrathin sections, Uranyl AcAntonescu, C.R.; Fletcher, C.D.M.; Kim, A.; Lazar, A.J.; Quezado, M.M.; Reilly, K.M.; Stemmer-Rachamimov, A.; Stewart, D.R.; Viskochil, D.; Widemann, B.; Perry, A. Histopathologic evaluation of atypical neurofibromatous tumors and their transformation into malignant peripheral nerve sheath tumor in patients with neurofibromatosis 1-a consensus overview. Hum. Pathol. 2017, 67, 1–10.
88. Ide, F.; Muramatate and Lead citsu, T.; Kikuchi, K.; Saito, I.; Kusama, K. Oral plexiform schwannoma with unusual epithelial induction. J. Cutan Pathol. 2015, 42, 978–982.
89. Sergherate. Bar: A, B, C, E, 80 µm; D, 60 µm; F, G, 0.5 µmert, J.; Zachar, D.; Furon, V.; Khonsari, R.H.; Ortonne, N.; Mauprivez, C. Oral plexiform schwannoma: A case report and relevant immunohistochemical investigation. SAGE Open Med. Case Rep. 2019, 7:2050313 × 19838184.