6. Podocyte Physiopathology and Infections
It is well known that, in the course of nephrotic syndrome, infections lead to an exacerbation of proteinuria
[55] and are an important risk factor for relapses
[1][56][57][1,56,57].
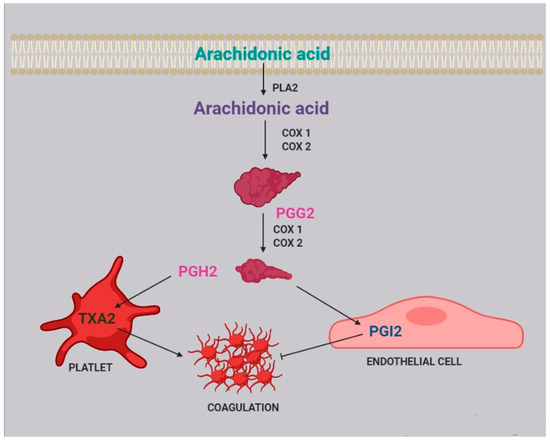
Infections activate the immune system, triggering the inflammatory cascade. It was recently reported that during inflammation two enzymes are induced: 15-lipoxygenase (15-LO) and secreted phospholipase A
2 (sPLA
2)
[58]. Notably, 15-LO is expressed in human podocytes
[59], while sPLA
2 is expressed in platelets, neutrophils, eosinophils, and macrophages
[60]. sPLA2 releases AA from membrane phospholipids
[51][61][51,61] acting in a paracrine way.
In glomerular podocytes, intracellular free AA is metabolized to PGE
2, which, by interacting with the EP 4 receptor (prostaglandin E
2 receptor 4) expressed by podocytes, reduces AA release
[52]. This loop regulates podocyte function in both physiological and pathological conditions and is able to change PGE
2 synthesis
[62].
As described above, during the course of an infection, intracellular AA levels increase due to the action of sPLA
2. It was reported that in podocytes, an excess of AA activates protein kinase A, which in turn promotes c-Abl activation and nephrin phosphorylation, thus causing actin cytoskeleton remodeling and podocyte injury
[63]. This mechanism could partially explain the frequent recurrence of proteinuria during infectious episodes in children. Moreover, an increase in sPLA
2 1B levels and PLA
2R expression has been observed to be positively associated to podocyte apoptosis in kidneys of patients with idiopathic membranous nephropathy
[64].
Podocyte foot process injury and podocyte apoptosis due to cytoskeleton remodeling were also attributed to a change of Ca
2+ efflux
[51], driven by 20-HETE, the main AA metabolite. It has also been observed that 20-HETE increases the current Ca
2+ flowing through TRPC6 channels in the podocyte
[51], which are located at the slit diaphragm, possibly leading to cellular injury.
7. Renal Fibrosis
Renal fibrosis is a process that progresses independently of the primary renal disease
[65] and represents a failed wound-healing process of the kidney tissue. Renal biopsies of patients with steroid-resistant nephrotic syndrome often show glomerulosclerosis and interstitial fibrosis, which are associated with progression to end-stage kidney disease in more than 50% of cases
[65], a poor prognosis that heightens the necessity of increasing our knowledge of the mechanisms underlying fibrosis.
Renal fibrosis is characterized by connective tissue deposition in the kidney parenchyma, particularly in the interstitial space and within the walls of glomerular capillaries, and by the consequent cellular processes. Fibrosis also interferes with normal tubular function, leading progressively to organ failure
[65][66][65,66].
The scar tissue contains fibrillar collagen I and III as well as some constituents of the normal capillary basement membrane, like collagen IV and V, fibronectin, laminin, perlecan, and heparin
[66].
Fibrosis is associated with leukocyte recruitment, angiogenesis, vascular leak, and the appearance of myofibroblasts. In particular, both the glomerulus and the interstitium attract large numbers of leukocytes, the majority of which are of myeloid lineage, and mostly neutrophiles in acute settings, whereas macrophages and dendritic cells predominate in chronic settings. In the case of chronic immune-mediated diseases, T lymphocytes are predominant
[66].
Activated macrophages may either damage the tissue directly or generate profibrotic cytokines, including TGF-β and other growth factors, and are capable of producing some matrix constituents. It is therefore evident that fibrosis and renal inflammation, primarily driven by immune system activation, are closely related.
Beyond its role in immune function regulation, AA is also directly related to fibrosis. In vitro experiments of cell cultures incubated with PUFAs showed that AA is able to induce upregulation of the expression of TGF-β, fibronectin 1 (FN1), connective tissue growth factor (CTGF), and collagen IV, all compounds related to fibrosis
[67]. AA also enhances in vitro angiotensin II (AngII)-induced gene expression
[67], activating mechanisms that mediate renal damage. Interestingly, omega-3 EPA and DHA, if administered with AA, suppress the effects of both AA and AngII
[67]. On the other side, angiotensin II is degraded to form angiotensin-(1-7), which inhibits angiotensin II-stimulated phosphorylation of the mitogen-activated protein kinases (MAPKs) p38, extracellular signal-related kinase (ERK1/ERK2), and C-JUN N-terminal kinase (JNK) in proximal tubular cells, thus exerting a protective role against fibrosis. As a matter of fact, the p38 MAPK phosphorylation leads to the release of AA and the production of TGF-β 1 and extracellular matrix proteins
[68].
20-HETE, an AA metabolite, also plays a distinct role in fibrogenesis, by activating the renin-angiotensin-aldosterone system (RAAS), by inducing vascular expression of ACE downstream of NF-κB activation
[69][70][69,70]. It is well known that the RAAS is involved in renal fibrosis
[71], because it increases TGF-β expression, which starts a biomolecular cascade driving to renal fibrosis.
On the contrary, PGE
2, another AA metabolite, has been shown to inhibit collagen type 1 production and to induce matrix metalloproteinase 1 (MMP1) expression in dermal fibroblasts
[72] by binding to the EP-1 receptor on fibroblasts, starting a pathway-regulated ERK1/2 and IP3 signaling that leads to a reduction in collagen expression and an increase in MMP1 expression
[72].
8. Drug and Gene Interactions
Idiopathic nephrotic syndrome is usually treated with glucocorticoids or with immunosuppressive drugs, particularly calcineurine inhibitors (CNI), such as cyclosporine A (CsA) and tacrolimus (Fk). CNIs are metabolized mainly by cytochrome P450, encoded by the CYP gene cluster. As seen above, the CYP gene is also involved in AA metabolism, but in the literature, there are no reports of enzymatic competition between these drugs and AA.
With regard to the relationship between CNI and AA blood levels, an in vitro study reported that CsA decreases the activity of Delta 9 desaturase and increases the activity of Delta 6 and Delta 5 desaturases
[73] through unknown mechanisms. However, as Delta 5 desaturase is involved in the last step of AA biosynthesis
[73], CsA therapy could increase AA blood level of patients with INS.
On the same line, it has been suggested that CsA mostly increased the availability of free AA instead of decreasing AA blood levels through the acceleration of AA conversion by the cyclooxygenase pathway
[74], but a further in vitro study concluded that CsA had no effect on AA release and metabolism
[75]. This result was confirmed more recently in a study of CsA and glucocorticosteroids in human peripheral blood mononuclear cells
[76].
With regard to the role of AA metabolism in determining CNI side effects, it is well known that CsA treatment may cause gingival overgrowth, which depends on PGE
2 production in gingival fibroblasts. In fact, CsA potentiates TNF-α to stimulate the release of AA from fibroblasts, with consequent enhanced production of PGE
2 and gingival overgrowth
[77]. There are no studies reporting the same effect in other tissues.
The nephrotoxicity of CsA is well established, and Fk administration is associated with the same side effect, which has been linked to CYP2C8*3 and CYP2C8*4 polymorphisms and a consequent reduction of EETs: it was observed that a circulating Fk plasma concentration of 10 ng/mL is able to reduce the production of eicosanoids by 35%. It follows that CNIs-induced nephrotoxicity could be due to a reduced activity of CYP2C8*3, which reduces the production of EETs, enhancing drug nephrotoxicity
[78].
Pre-treatment with Fk is also known to enhance glucocorticoids to inhibit AA and PGE
2 production
[79] by inhibiting COX2 expression, but the co-administration of Fk and glucocorticoids does not inhibit COX2 expression, allowing for normal PGE
2 production
[79].
9. Dietary Balance Between AA and LA and AA Sources
AA, which belongs to the omega-6 series, and docosahexaenoic acid (DHA), which belongs to the omega-3 series, are the most important byproducts of essential fatty acids linoleic and α-linolenic acid, and their imbalance has been associated with inflammatory and chronic disorders
[80][82].
While LA and AA are mostly known as inflammatory molecules, operating within an interdependent network through their metabolites
[80][82], AA metabolites also have anti-inflammatory and protective roles, while LA metabolites affect immune function by binding cellular receptors and altering signaling molecules
[81][83].
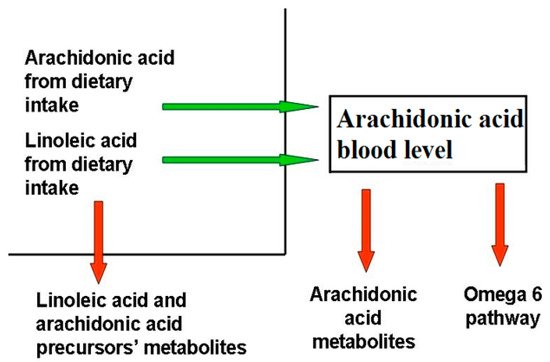
AA and DHA levels depend on both to genetic predisposition and diet intake. Blood AA levels, as shown in , can be modulated through dietary habits, taking into account that there is a marked difference between the amount of AA supplied with the diet and the amount synthesized by human metabolic pathways. In the latter case, the main rate-limiting enzymes are the Δ5- and Δ6-desaturases, which are encoded by the genes FADS1 and FADS2, and different polymorphisms in the fatty acid desaturases genes might even increase or decrease the production of these LC-PUFAs
[82][84]. As a matter of fact, looking at the frequencies of the 28 SNPs in the FADS haplotypes, their distribution in the 3 main haplotypes is evident over the world
[83][85].
Unlike other fatty acids, omega-3 and omega-6 precursors (LA and linolenic acid, respectively) cannot be synthesized de novo by mammals (they are essential dietary compounds indeed), so the relative abundance of these PUFAs in the diet has a major influence in humans.
LA is the most represented omega-6 PUFA in most western diets, and is widely distributed in foods: it represents more than 50% of the lipid content in various vegetable oils, including safflower, sunflower, corn, and soybean oils; it is present in high amounts in nuts and seeds, while lower levels are found in whole grains, legumes, some meats, eggs, and dairy products
[84][86]. Notably, it was recently reported that a strong reduction in dietary intake of LA was not associated with a linear decrease in circulating AA levels
[85][87].
The AA state depends on the endogenous synthesis from the essential precursor LA, undergoing desaturation and elongation, and the direct dietary intake
[86][88]. Since LA to AA conversion efficiency is low in humans, AA intake through the diet appears to be significantly more effective in raising its circulating levels.