Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Peter Tang and Version 1 by MARYAM AZLAN.
Osteoarthritis (OA) is a chronic degenerative disorder of the joint and its prevalence and severity is increasing owing to ageing of the population. Osteoarthritis is characterized by the degradation of articular cartilage and remodeling of the underlying bone. Extracellular vesicles are naturally released by cells and they carry their origin cell information to be delivered to target cells. On the other hand, mesenchymal stem cells (MSCs) are highly proliferative and have a great potential in cartilage regeneration.
- extracellular vesicles
- exosomes
- mesenchymal stem cells
- osteoarthritis
- chondrocytes
1. Introduction
Osteoarthritis (OA) is a common degenerative disorder of the joints that affects the knee, hands, hip, spine and feet. Osteoarthritis represents a large and increasing health burden, causing patients’ function deterioration as well as extending public health costs to deal with an increasing OA prevalence worldwide. According to data from the United States Center for Disease Control and Prevention, OA affected 52.5 million people in the US in 2012 and is expected to affect 78 million people by 2040 [1]. In 2017, OA accounts for approximately 7.1% of the musculoskeletal disorders burden and showed a statistically significant increase in comparison to 31.4% in 2007 (95% CI:30.7,32.1) [2,3][2][3]. Meanwhile, between 1990 to 2019, the number of people affected globally by OA increased by 48% [4].
The prevalence of OA is increasing due to obesity and lifestyle. Patients with OA have limited daily activities and a greater risk of mortality [5]. Age is the main factor for OA due to ageing, muscle weakness and thinning of cartilage. Meanwhile, obesity is strongly associated with knee OA [6]. Apart from that, genetics and diet also influence the risk of OA. To date, the treatment for OA involves pain management using non-steroid anti-inflammatory drugs (NSAIDs) and pain killer to relive the symptoms [7] and surgical therapy for end-stage OA patients [8]. However, surgical therapy is costly and may lead to tissue hypertrophy. Therefore, appropriate therapeutic strategies are crucial to overcome existing problems. Recently, mesenchymal stem cells (MSCs)-derived extracellular vesicles (EV) therapy has been suggested as a potential therapeutic strategy for OA in an attempt to replace invasive treatments. MSC-derived EV is safe and a promising therapy due to their unique properties including a small size, stable culture, low immunogenicity, specific targeting and carrying biologically active components which make them a potential natural therapeutic delivery agent [9]. The small size of MSC-derived EV permits them to pass through cell barriers easily, thus increasing their capability in delivering genetic materials directly into the cytoplasm of the recipient cells [10,11][10][11].
2. Pathophysiology of OA
Osteoarthritis is a progressive chronic condition that represents a pathological imbalance of degradative and reparative processes involving the entire joint and its component parts, with secondary inflammatory changes, particularly in the synovium as well as in the articular cartilage [12]. This complex process of disease involves biomechanical changes in joint composition, inflammation of the joints and metabolic changes which consequently lead to abnormal equilibrium of the synovial joint [13]. The definition and terminology of OA have long been a debatable subject centered on articular cartilage that loses its integrity [14]. Osteoarthritis is an active dynamic alteration arising from an imbalance between degradation and regeneration of diarthrodial joint tissues involving hyaline articular cartilage, subchondral bone, cruciate and collateral ligaments, capsule membrane and synovial membrane [15,16][15][16]. The key pathophysiological mechanisms in OA involve pro-inflammatory cytokines (interleukins (IL)-1, IL-6, IL-8 and tumor necrosis factor (TNF)-α) and pro-catabolic mediators through their signaling pathway and the well-characterized effect of nuclear factor κB (NFκB) and mitogen-activated protein kinase (MAPK) signaling responses and reprogramming are switching pathways in transcriptional networks (Figure 1) [17].
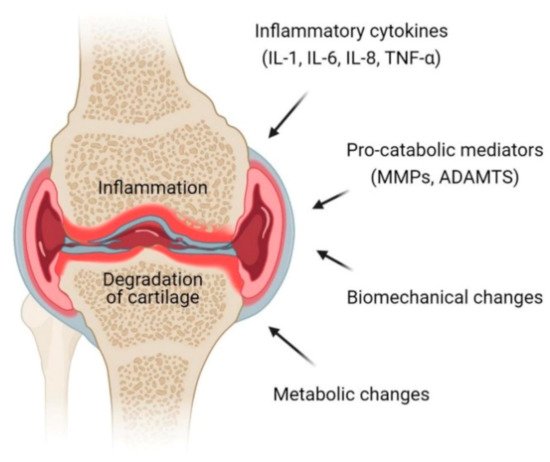
Figure 1. Pathophysiology of osteoarthritis (OA) at the knee joint. Inflammation and degradation of cartilage at the joint are common features of OA which resulted from the release of proinflammatory cytokines including interleukin (IL)-1, IL-6, IL-8 and TNF-α, pro-catabolic mediators such as matrix metalloproteinases (MMPs) and a disintegrin and metalloproteinase with thrombospondin motifs (ADAMTS), biomechanical changes and metabolic changes.
Cartilage consists of chondrocytes that produce a large amount of extracellular matrix. Chondrocytes in a healthy articular cartilage resist proliferation and differentiation, while chondrocytes in diseased cartilage proliferate and develop hypertrophy. The inflammatory mediators, mechanical and oxidative stress compromise the function and viability of chondrocytes, reprogramming them to undergo hypertrophic differentiation and early senescence, making them even more sensitive to the effects of pro-inflammatory and pro-catabolic mediators [18]. Products that are released from the cartilage matrix and chondrocytes in response to adverse mechanical forces and other factors induce the release of products that deregulate chondrocyte function via paracrine and autocrine mechanisms.
3. Treatment of OA
3.1. Osteoarthritis Management and Current Therapy
In view of the complexity of OA mechanism, a comprehensive plan for management of OA in different patients may include awareness in terms of educational plan, behavioral, psychosocial and physical interventions, as well as pharmacological treatment such as the use of topical, oral and intra-articular injection and also surgical treatment [19]. The principle of treatment should be addressed according to the degree of severity as well as the patient’s medical status to ensure that a personalized management strategy is tailored to their needs. The goals are to reduce symptoms and ultimately slow the disease progression, which may in turn improve quality of life and consequently reduce the health cost burden.
3.2. Pharmacological and Non-Pharmacological Therapy
A multidisciplinary, patient-centered combination of education, self-management, exercise, weight loss with realistic goals, encouragement and regular reassessment is recommended for individuals with OA [20]. Commonly, topical NSAIDs, oral NSAIDs and tramadol are used to treat OA. However, inconclusive evidence has been shown for acetaminophen, non-tramadol opioids and intra articular injections of corticosteroids, hyaluronic acid (HA) and platelets rich plasma (PRP) [21]. Clinical trials of intra articular glucocorticoid injections have demonstrated a short-term efficacy in knee OA as well as for hand OA [19]. However, a recent report raised the possibility that specific steroid preparations or a certain frequency of steroid injections may contribute to cartilage loss; however, the clinical significance of this finding was uncertain, particularly since change in cartilage thickness was not associated with a worsening in pain, function or other radiographic features [22].
Surgical intervention is indicated in end stage OA when all previous treatment methods have failed, a significant loss in quality of life and when the patient is severely symptomatic. Surgery is an effective treatment option; however, the long-term success rate is not clear and may fail depending on the longevity of the implant. Surgical techniques may include osteotomy around the knee, unicondylar arthroplasty (partial knee replacement) or total knee arthroplasty based on the patients’ condition and degree of deformity or joint destruction [23].
4. Mesenchymal Stem Cells
4.1. The Source of Mesenchymal Stem Cells, Isolation and Characterization
Mesenchymal stem cells (MSCs) were first discovered in 1966 from the bone marrow [24] and hold the concept from postnatal progenitor [25]. They are also known as multipotent mesenchymal stromal cells which possess two major features, including the ability to differentiate into multiple lineages and the capacity of self-renewal. According to the International Society for Cellular Therapy (ISCT), MSCs must meet three minimal criteria [26]. Firstly, MSCs must exhibit plastic-adherence when grown in vitro. Secondly, MSCs must express the surface antigens CD73, CD90 and CD105 while lacking expression of CD45, CD34, CD14 or CD11b, CD79α or CD19 and HLA-DR. Thirdly, MSCs must be able to differentiate into mesodermal cell types (i.e., adipocytes, chondrocytes and osteoblasts) when cultured under specific conditions.
Mesenchymal stem cells can be isolated from various tissues and are not restricted to mesodermal origin-type of cells such as bone marrow, adipose, muscle or bone. They were originally found in the bone marrow [24], but later studies identified MSCs in other tissues such as in the peripheral blood, brain, spleen, liver, kidney, lung, thymus, placental, umbilical cord and pancreas. Mesenchymal stem cells share a similar characteristic phenotype [26] despite their presence in different tissue sources, albeit with some additional features that represent their tissue origin [27]. Although MSCs can be isolated from almost every type of connective tissues [28], studies have shown that bone marrow represents the major source of MSCs [29,30,31][29][30][31].
4.2. Mesenchymal Stem Cell-Based Therapy
Mesenchymal stem cells have become a popular cell source for therapeutic purposes due to their immunomodulation and regenerative properties [32,33,34,35,36,37,38][32][33][34][35][36][37][38]. Mesenchymal stem cells possess the capacity to migrate to injured sites in response to other cells and environmental signals and also promote tissue regeneration orchestrated by the paracrine secretion of a broad repertoire of growth factors, chemokines and cytokines [39]. Through interaction with the host niche, MSCs were able to secrete bioactive mediators, such as growth factors, cytokines and EV that exert immunosuppressive, anti-apoptotic, anti-fibrotic, angiogenic and anti-inflammatory effects [40,41][40][41]. In addition, MSCs exhibited immunomodulatory functions by preventing immune cells’ activation or proliferation [42]. The immunomodulatory function of MSCs make them a good option for cell-based therapy, as the possibility for cell rejection is reduced.
The ability of MSCs to differentiate into various cell types enables their use as a tool in regenerative medicine. Mesenchymal stem cells have been reported to be successfully used as a therapy against many diseases and clinical conditions. At present, there are over 950 clinical trials worldwide that have used MSCs to treat various diseases [43], including bone and cartilage repair, diabetes, cardiovascular diseases, liver disease, immune-related, neurodegenerative diseases and spinal cord injuries. Mesenchymal stem cells have shown promising results in the clinical application of cell-based therapy. The efficacy of MSCs on bone regeneration in various orthopedic conditions has been widely demonstrated. Several clinical trials at different phases (I, II or III) have been performed for bone fracture repair using various sources of MSCs which were implanted either via direct injection or incorporated with osteogenic matrix or scaffolds which promoted bone repair and functions [44,45,46][44][45][46].
4.3. Mesenchymal Stem Cell-Based Therapy in Osteoarthritis Treatment
Regenerative medicine has become increasingly popular as a promising new approach for OA since articular cartilage has a limited capacity for spontaneous intrinsic repair, and may progress to OA, owing to the sparse distribution of highly differentiated chondrocytes, the low supply of progenitor cells and the lack of vascular supply [47]. This is evidenced by wide availability in clinical practice and by publications of many case series and clinical trials.
Cell-based therapy has been a promising option in OA because it is aimed at reversing the symptoms and pathophysiology of OA [48]. The ability of MSCs to differentiate between multiple lineages including musculoskeletal tissue supports the use of MSCs as an excellent source for degenerative musculoskeletal conditions such as in OA [49]. The therapeutic potential of MSCs in the treatment of OA is aimed at cartilage repair and restoration (Figure 2) [50].
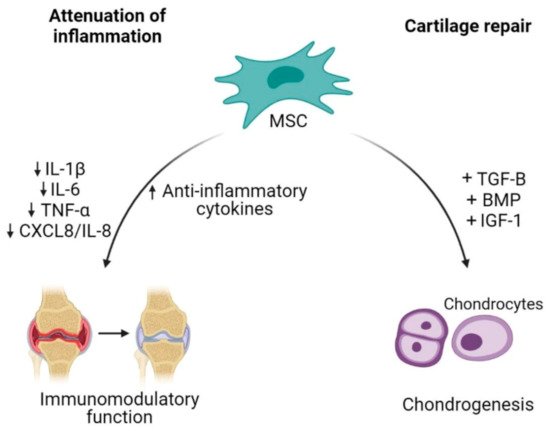
Figure 2. Mesenchymal stem cells (MSCs) as an alternative cell-based therapy for osteoarthritis. Mesenchymal stem cells dampen inflammatory activities in OA by reducing the releases of pro-inflammatory cytokines and increasing anti-inflammatory cytokines. Additionally, their plasticity characteristic allows differentiation of MSCs into chondrocytes, thus contributing to cartilage repair.
The capability of MSCs to undergo chondrogenic differentiation assists in the regeneration of injured cartilage. In order to successfully enhance MSCs differentiation into chondrocytes, MSCs require soluble factors to promote differentiation such as transforming growth factor (TGF)-β, bone morphogenetic protein (BMP) and insulin growth factor (IGF)-1 [51,52][51][52]. The combination of BPM2/TGF-β in pre-chondrogenic medium resulted in high expression of chondrocytes specific genes such as collagen type II alpha 1 (COL2A1) and aggrecan (ACAN) [53]. Mesenchymal stem cells proliferation could also be enhanced by a low oxygen condition, which results in an increase of cartilage specific genes expression (COL2A1 and SOX9) and proteoglycan synthesis during chondrogenic differentiation [54]. Besides being able to regenerate or restore injured cartilage, MSC-based therapy also focuses on the inflammation attenuation. MSCs were shown to orchestrate immunomodulatory function of inflammatory responses through paracrine activities [55]. Adipose tissue-derived MSCs co-cultured with chondrocytes or synoviocytes showed a decrease in the expression of inflammatory factors such as IL-1β, TNF-α, IL-6 and CXCL8/IL-8 through the COX-2/PGE2 pathway as modulators, exerting anti-inflammatory effects in OA condition [56].
There were several strategies used by manipulating different MSCs sources and conditions for potential OA treatment [57]. Different sources of MSCs contribute to different outcomes in cartilage regeneration. For example, human skeletal stem cells (hSSCs) have shown the ability to generate into multilineage ossicles containing bone, cartilage and stroma, which have a potential for chondrogenic and osteogenic activities [58]. Apart from selecting different sources of MSCs, manipulating the MSCs in an in vitro culture condition also enhances chondrogenesis. A co-culture of human chondrocytes and chondro-progenitors (also known as cartilage cells) resulted in increased chondrogenic ability and increased cytokines and growth factors compared to bone-marrow derived MSCs [59]. Furthermore, controlling the culture condition also contributes to better chondrogenic differentiation such as culturing MSCs with TGF-β [60] or fibroblast growth factor (FGF)-2, 9 and 18 [61].
Direct intra articular injection of MSCs has been shown to improve the condition of OA patients [62]. Several studies have reported the use of intra articular injection of autologous bone-marrow-derived MSCs, which showed improvement in clinical symptoms and resulted in higher arthroscopic and histological grades than the control group [63,64][63][64]. In another study, an intra articular injection of autologous MSCs showed significant growth of cartilage and meniscus on magnetic resonance imaging (MRI), increased range of motion and modified visual analog scale (VAS) pain scores at 24-weeks post injection [65]. Intra articular injection of autologous bone marrow-derived MSCs showed significant improvement in terms of knee pain and quality of life during the 6-month follow-up [66].
Articular cartilage tissue engineering is another aspect that can be manipulated by using biomaterial constructs. Several biomaterials such as fibrin [67], biopolymer (chitosan) [68], synthetic polymers [69] and hydrogel [70] were widely used for their ability to degrade rapidly, their low immunogenicity and their ability to enhance MSCs proliferation and chondrogenic differentiation.
Another strategy to achieve chondrogenic differentiation of MSCs is via extracellular matrix (ECM) application by using synthetic or natural scaffolds. MSCs cultured in vitro in serum free conditions or in hydrogel or scaffold materials such as polymers, alginate beads and collagen sponges will eventually promote the differentiation of MSCs into chondrocytes [71]. Early pre-clinical studies have showed promising chondrogenic differentiation properties when MSCs were seeded on scaffolds [72,73][72][73]. Autologous transplantation of rabbit MSCs with HA gel sponges showed a well-repaired cartilage tissue which resembles the articular cartilage of the surrounding structure [73]. Another study has also used intra-articular injection of autologous adipose MSCs combined with HA which suppressed the OA progression and promoted cartilage regeneration [74]. The first autologous bone marrow-derived MSCs transplant embedded in vitro in a collagen gel was reported in 2004. A year later, the use of the matrix-induced autologous chondrocyte implantation (MACI) technique was reported by using a collagen bilayer seeded with chondrocytes [75]. Since then, many studies reported promising findings by using the MACI technique with various types of scaffolds, especially collagen due to its natural presence in the ECM [76]. Articular cartilage regeneration using allogeneic human umbilical cord blood-derived MSCs which were expanded in HA hydrogel demonstrated production of hyaline-like cartilage after one year and the regenerated cartilage persisted after three years [77].
The use of MSCs for articular cartilage regeneration has several limitations. A transcriptome analyses of human neonatal articular cartilage (hNAC) and MSC-derived cartilage has demonstrated that over 500 genes that are highly expressed in hNAC were not expressed in MSC chondrogenesis [78]. This suggests that in vitro MSC-derived cartilage may not represent the actual in vivo chondrocytes. In addition, in vitro cultured MSCs displayed a hypertrophic phenotype, thus limiting their application in articular cartilage tissue engineering [79]. Since MSCs are heterogenous, their numbers vary in tissues and different MSC sources may not be equivalent in terms of function [79,80][79][80].
References
- Hootman, J.M.; Helmick, C.G.; Barbour, K.E.; Theis, K.A.; Boring, M.A.J.A. Rheumatology. Updated projected prevalence of self-reported doctor-diagnosed arthritis and arthritis-attributable activity limitation among US adults, 2015–2040. Arthritis Rheumatol. 2016, 68, 1582–1587.
- Kloppenburg, M.; Berenbaum, F.J.O. Osteoarthritis year in review 2019: Epidemiology and therapy. Osteoarthr. Cartil. 2020, 28, 242–248.
- Vos, T.; Allen, C.; Arora, M.; Barber, R.M.; Bhutta, Z.A.; Brown, A.; Carter, A.; Casey, D.C.; Charlson, F.J.; Chen, A.Z.J.T.l. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016, 388, 1545–1602.
- Hunter, D.J.; March, L.; Chew, M. Osteoarthritis in 2020 and beyond: A Lancet Commission. Lancet 2020, 396, 1711–1712.
- Palazzo, C.; Nguyen, C.; Lefevre-Colau, M.M.; Rannou, F.; Poiraudeau, S. Risk factors and burden of osteoarthritis. Ann. Phys. Rehabil. Med. 2016, 59, 134–138.
- Silverwood, V.; Blagojevic-Bucknall, M.; Jinks, C.; Jordan, J.L.; Protheroe, J.; Jordan, K.P. Current evidence on risk factors for knee osteoarthritis in older adults: A systematic review and meta-analysis. Osteoarthr. Cartil. 2015, 23, 507–515.
- Rannou, F.; Pelletier, J.-P.; Martel-Pelletier, J. Efficacy and safety of topical NSAIDs in the management of osteoarthritis: Evidence from real-life setting trials and surveys. Semin. Arthritis Rheum. 2016, 45, S18–S21.
- Okoro, T.; Morrison, V.; Maddison, P.; Lemmey, A.B.; Andrew, J.G. An assessment of the impact of behavioural cognitions on function in patients partaking in a trial of early home-based progressive resistance training after total hip replacement surgery. Disabil. Rehabil. 2013, 35, 2000–2007.
- Li, J.J.; Hosseini-Beheshti, E.; Grau, G.E.; Zreiqat, H.; Little, C.B. Stem cell-derived extracellular vesicles for treating joint injury and osteoarthritis. Nanomaterials 2019, 9, 261.
- Jiang, L.; Vader, P.; Schiffelers, R.M. Extracellular vesicles for nucleic acid delivery: Progress and prospects for safe RNA-based gene therapy. Gene Ther. 2017, 24, 157–166.
- Mehrotra, N.; Tripathi, R.M. Short interfering RNA therapeutics: Nanocarriers, prospects and limitations. IET Nanobiotechnol. 2015, 9, 386–395.
- Martel-Pelletier, J.; Barr, A.J.; Cicuttini, F.M.; Conaghan, P.G.; Cooper, C.; Goldring, M.B.; Goldring, S.R.; Jones, G.; Teichtahl, A.J.; Pelletier, J.-P. Osteoarthritis. Nat. Rev. Dis. Primers 2016, 2, 16072.
- Hunter, D.J.; Bierma-Zeinstra, S. Osteoarthritis. Lancet 2019, 393, 1745–1759.
- Loeser, R.F.; Collins, J.A.; Diekman, B.O. Ageing and the pathogenesis of osteoarthritis. Nat. Rev. Rheumatol. 2016, 12, 412–420.
- Fu, K.; Robbins, S.R.; McDougall, J.J. Osteoarthritis: The genesis of pain. Rheumatology 2018, 57, iv43–iv50.
- Prieto-Alhambra, D.; Arden, N.; Hunter, D.J. Osteoarthritis: The Facts, 2nd ed.; OUP Oxford: Oxford, UK, 2014.
- Liu-Bryan, R.; Terkeltaub, R. Emerging regulators of the inflammatory process in osteoarthritis. Nat. Rev. Rheumatol. 2015, 11, 35.
- Mobasheri, A.; Batt, M. An update on the pathophysiology of osteoarthritis. Ann. Phys. Rehabil. Med. 2016, 59, 333–339.
- Kolasinski, S.L.; Neogi, T.; Hochberg, M.C.; Oatis, C.; Guyatt, G.; Block, J.; Callahan, L.; Copenhaver, C.; Dodge, C.; Felson, D.J.A.; et al. 2019 American College of Rheumatology/Arthritis Foundation guideline for the management of osteoarthritis of the hand, hip, and knee. Arthritis Rheumatol. 2020, 72, 220–233.
- Fernandes, L.; Hagen, K.B.; Bijlsma, J.W.; Andreassen, O.; Christensen, P.; Conaghan, P.G.; Doherty, M.; Geenen, R.; Hammond, A.; Kjeken, I.; et al. EULAR recommendations for the non-pharmacological core management of hip and knee osteoarthritis. Ann. Rheum. Dis. 2013, 72, 1125–1135.
- Jevsevar, D.S. Treatment of osteoarthritis of the knee: Evidence-based guideline, 2nd edition. J. Am. Acad. Orthop. Surg. 2013, 21, 571–576.
- McAlindon, T.E.; LaValley, M.P.; Harvey, W.F.; Price, L.L.; Driban, J.B.; Zhang, M.; Ward, R.J.J.J. Effect of intra-articular triamcinolone vs saline on knee cartilage volume and pain in patients with knee osteoarthritis: A randomized clinical trial. JAMA 2017, 317, 1967–1975.
- Liu, C.Y.; Li, C.D.; Wang, L.; Ren, S.; Yu, F.B.; Li, J.G.; Ma, J.X.; Ma, X.L. Function scores of different surgeries in the treatment of knee osteoarthritis: A PRISMA-compliant systematic review and network-meta analysis. Medicine 2018, 97, e10828.
- Friedenstein, A.J.; Chailakhjan, R.K.; Lalykina, K.S. The development of fibroblast colonies in monolayer cultures of guinea-pig bone marrow and spleen cells. Cell Tissue Kinet. 1970, 3, 393–403.
- Bianco, P.; Robey, P.G.; Simmons, P.J. Mesenchymal stem cells: Revisiting history, concepts, and assays. Cell Stem Cell 2008, 2, 313–319.
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317.
- Hass, R.; Kasper, C.; Böhm, S.; Jacobs, R. Different populations and sources of human mesenchymal stem cells (MSC): A comparison of adult and neonatal tissue-derived MSC. Cell Commun. Signal. 2011, 9, 12.
- da Silva Meirelles, L.; Chagastelles, P.C.; Nardi, N.B. Mesenchymal stem cells reside in virtually all post-natal organs and tissues. J. Cell Sci. 2006, 119, 2204–2213.
- Noort, W.A.; Scherjon, S.A.; Kleijburg-van der Keur, C.; Kruisselbrink, A.B.; van Bezooijen, R.L.; Beekhuizen, W.; Willemze, R.; Kanhai, H.H.; Fibbe, W.E. Mesenchymal stem cells in human second-trimester bone marrow, liver, lung, and spleen exhibit a similar immunophenotype but a heterogeneous multilineage differentiation potential. Haematologica 2003, 88, 845–852.
- Wexler, S.A.; Donaldson, C.; Denning-Kendall, P.; Rice, C.; Bradley, B.; Hows, J.M. Adult bone marrow is a rich source of human mesenchymal ‘stem’ cells but umbilical cord and mobilized adult blood are not. Br. J. Haematol. 2003, 121, 368–374.
- Zeddou, M.; Briquet, A.; Relic, B.; Josse, C.; Malaise, M.G.; Gothot, A.; Lechanteur, C.; Beguin, Y. The umbilical cord matrix is a better source of mesenchymal stem cells (MSC) than the umbilical cord blood. Cell Biol. Int. 2010, 34, 693–701.
- Connick, P.; Kolappan, M.; Crawley, C.; Webber, D.J.; Patani, R.; Michell, A.W.; Du, M.-Q.; Luan, S.-L.; Altmann, D.R.; Thompson, A.J.; et al. Autologous mesenchymal stem cells for the treatment of secondary progressive multiple sclerosis: An open-label phase 2a proof-of-concept study. Lancet Neurol. 2012, 11, 150–156.
- Götherström, C.; Westgren, M.; Shaw, S.W.; Aström, E.; Biswas, A.; Byers, P.H.; Mattar, C.N.; Graham, G.E.; Taslimi, J.; Ewald, U.; et al. Pre- and postnatal transplantation of fetal mesenchymal stem cells in osteogenesis imperfecta: A two-center experience. Stem Cells Transl. Med. 2014, 3, 255–264.
- Karantalis, V.; DiFede, D.L.; Gerstenblith, G.; Pham, S.; Symes, J.; Zambrano, J.P.; Fishman, J.; Pattany, P.; McNiece, I.; Conte, J.; et al. Autologous mesenchymal stem cells produce concordant improvements in regional function, tissue perfusion, and fibrotic burden when administered to patients undergoing coronary artery bypass grafting: The Prospective Randomized Study of Mesenchymal Stem Cell Therapy in Patients Undergoing Cardiac Surgery (PROMETHEUS) trial. Circ. Res. 2014, 114, 1302–1310.
- Rushkevich, Y.N.; Kosmacheva, S.M.; Zabrodets, G.V.; Ignatenko, S.I.; Goncharova, N.V.; Severin, I.N.; Likhachev, S.A.; Potapnev, M.P. The use of autologous mesenchymal stem cells for cell therapy of patients with amyotrophic lateral sclerosis in belarus. Bull. Exp. Biol. Med. 2015, 159, 576–581.
- Thakkar, U.G.; Trivedi, H.L.; Vanikar, A.V.; Dave, S.D. Insulin-secreting adipose-derived mesenchymal stromal cells with bone marrow-derived hematopoietic stem cells from autologous and allogenic sources for type 1 diabetes mellitus. Cytotherapy 2015, 17, 940–947.
- Vega, A.; Martín-Ferrero, M.; Canto, F.; Alberca, M.; Garcia, V.; Munar, A.; Orozco, L.; Soler, R.; Fuertes, J.; Huguet, M.; et al. Treatment of knee osteoarthritis with allogeneic bone marrow mesenchymal stem cells: A randomized controlled trial. Transplantation 2015.
- Fernández, O.; Izquierdo, G.; Fernández, V.; Leyva, L.; Reyes, V.; Guerrero, M.; León, A.; Arnaiz, C.; Navarro, G.; Páramo, M.D.; et al. Adipose-derived mesenchymal stem cells (AdMSC) for the treatment of secondary-progressive multiple sclerosis: A triple blinded, placebo controlled, randomized phase I/II safety and feasibility study. PLoS ONE 2018, 13, e0195891.
- Yao, Y.; Huang, J.; Geng, Y.; Qian, H.; Wang, F.; Liu, X.; Shang, M.; Nie, S.; Liu, N.; Du, X.; et al. Paracrine action of mesenchymal stem cells revealed by single cell gene profiling in infarcted murine hearts. PLoS ONE 2015, 10, e0129164.
- Fan, X.L.; Zhang, Z.; Ma, C.Y.; Fu, Q.L. Mesenchymal stem cells for inflammatory airway disorders: Promises and challenges. Biosci. Rep. 2019, 39.
- Caplan, A.I.; Dennis, J.E. Mesenchymal stem cells as trophic mediators. J. Cell Biochem. 2006, 98, 1076–1084.
- Mundra, V.; Gerling, I.C.; Mahato, R.I. Mesenchymal stem cell-based therapy. Mol. Pharm. 2013, 10, 77–89.
- National Institutes of Health. Available online: (accessed on 13 October 2020).
- Ho-Shui-Ling, A.; Bolander, J.; Rustom, L.E.; Johnson, A.W.; Luyten, F.P.; Picart, C. Bone regeneration strategies: Engineered scaffolds, bioactive molecules and stem cells current stage and future perspectives. Biomaterials 2018, 180, 143–162.
- Calabrese, G.; Giuffrida, R.; Forte, S.; Fabbi, C.; Figallo, E.; Salvatorelli, L.; Memeo, L.; Parenti, R.; Gulisano, M.; Gulino, R. Human adipose-derived mesenchymal stem cells seeded into a collagen-hydroxyapatite scaffold promote bone augmentation after implantation in the mouse. Sci. Rep. 2017, 7, 7110.
- Gómez-Barrena, E.; Rosset, P.; Lozano, D.; Stanovici, J.; Ermthaller, C.; Gerbhard, F. Bone fracture healing: Cell therapy in delayed unions and nonunions. Bone 2015, 70, 93–101.
- McIntyre, J.A.; Jones, I.A.; Han, B.; Vangsness, C.T., Jr. Intra-articular mesenchymal stem cell therapy for the human joint: A systematic review. Am. J. Sports Med. 2018, 46, 3550–3563.
- Kong, L.; Zheng, L.Z.; Qin, L.; Ho, K.K.W. Role of mesenchymal stem cells in osteoarthritis treatment. J. Orthop. Transl. 2017, 9, 89–103.
- Wyles, C.C.; Houdek, M.T.; Behfar, A.; Sierra, R.J. Mesenchymal stem cell therapy for osteoarthritis: Current perspectives. Stem Cells Cloning 2015, 8, 117–124.
- Nöth, U.; Steinert, A.F.; Tuan, R.S. Technology insight: Adult mesenchymal stem cells for osteoarthritis therapy. Nat. Clin. Pract. Rheumatol. 2008, 4, 371–380.
- Yu, D.A.; Han, J.; Kim, B.S. Stimulation of chondrogenic differentiation of mesenchymal stem cells. Int. J. Stem Cells 2012, 5, 16–22.
- Puetzer, J.L.; Petitte, J.N.; Loboa, E.G. Comparative review of growth factors for induction of three-dimensional in vitro chondrogenesis in human mesenchymal stem cells isolated from bone marrow and adipose tissue. Tissue Eng. Part B Rev. 2010, 16, 435–444.
- Ronzière, M.C.; Perrier, E.; Mallein-Gerin, F.; Freyria, A.M. Chondrogenic potential of bone marrow- and adipose tissue-derived adult human mesenchymal stem cells. Bio-Med. Mater. Eng. 2010, 20, 145–158.
- Bae, H.C.; Park, H.J.; Wang, S.Y.; Yang, H.R.; Lee, M.C.; Han, H.-S. Hypoxic condition enhances chondrogenesis in synovium-derived mesenchymal stem cells. Biomater. Res. 2018, 22, 28.
- Glenn, J.D.; Whartenby, K.A. Mesenchymal stem cells: Emerging mechanisms of immunomodulation and therapy. World J. Stem Cells 2014, 6, 526–539.
- Manferdini, C.; Maumus, M.; Gabusi, E.; Piacentini, A.; Filardo, G.; Peyrafitte, J.A.; Jorgensen, C.; Bourin, P.; Fleury-Cappellesso, S.; Facchini, A.; et al. Adipose-derived mesenchymal stem cells exert antiinflammatory effects on chondrocytes and synoviocytes from osteoarthritis patients through prostaglandin E2. Arthritis Rheum. 2013, 65, 1271–1281.
- Colombini, A.; Perucca Orfei, C.; Kouroupis, D.; Ragni, E.; De Luca, P.; ViganÒ, M.; Correa, D.; de Girolamo, L. Mesenchymal stem cells in the treatment of articular cartilage degeneration: New biological insights for an old-timer cell. Cytotherapy 2019, 21, 1179–1197.
- Chan, C.K.F.; Gulati, G.S.; Sinha, R.; Tompkins, J.V.; Lopez, M.; Carter, A.C.; Ransom, R.C.; Reinisch, A.; Wearda, T.; Murphy, M.; et al. Identification of the human skeletal stem cell. Cell 2018, 175, 43–56.e21.
- De Luca, P.; Kouroupis, D.; Viganò, M.; Perucca-Orfei, C.; Kaplan, L.; Zagra, L.; de Girolamo, L.; Correa, D.; Colombini, A. Human diseased articular cartilage contains a mesenchymal stem cell-like population of chondroprogenitors with strong immunomodulatory responses. J. Clin. Med. 2019, 8, 423.
- Giuliani, N.; Lisignoli, G.; Magnani, M.; Racano, C.; Bolzoni, M.; Dalla Palma, B.; Spolzino, A.; Manferdini, C.; Abati, C.; Toscani, D.; et al. New insights into osteogenic and chondrogenic differentiation of human bone marrow mesenchymal stem cells and their potential clinical applications for bone regeneration in pediatric orthopaedics. Stem Cells Int. 2013, 2013, 312501.
- Correa, D.; Somoza, R.A.; Lin, P.; Greenberg, S.; Rom, E.; Duesler, L.; Welter, J.F.; Yayon, A.; Caplan, A.I. Sequential exposure to fibroblast growth factors (FGF) 2, 9 and 18 enhances hMSC chondrogenic differentiation. Osteoarthr. Cartil. 2015, 23, 443–453.
- Lopa, S.; Colombini, A.; Moretti, M.; de Girolamo, L. Injective mesenchymal stem cell-based treatments for knee osteoarthritis: From mechanisms of action to current clinical evidences. Knee Surg. Sports Traumatol. Arthrosc. Off. J. ESSKA 2019, 27, 2003–2020.
- Wakitani, S.; Nawata, M.; Tensho, K.; Okabe, T.; Machida, H.; Ohgushi, H. Repair of articular cartilage defects in the patello-femoral joint with autologous bone marrow mesenchymal cell transplantation: Three case reports involving nine defects in five knees. J. Tissue Eng. Regen. Med. 2007, 1, 74–79.
- Wakitani, S.; Mitsuoka, T.; Nakamura, N.; Toritsuka, Y.; Nakamura, Y.; Horibe, S. Autologous bone marrow stromal cell transplantation for repair of full-thickness articular cartilage defects in human patellae: Two case reports. Cell Transplant. 2004, 13, 595–600.
- Centeno, C.J.; Busse, D.; Kisiday, J.; Keohan, C.; Freeman, M.; Karli, D. Increased knee cartilage volume in degenerative joint disease using percutaneously implanted, autologous mesenchymal stem cells. Pain Physician 2008, 11, 343–353.
- Garay-Mendoza, D.; Villarreal-Martínez, L.; Garza-Bedolla, A.; Pérez-Garza, D.M.; Acosta-Olivo, C.; Vilchez-Cavazos, F.; Diaz-Hutchinson, C.; Gómez-Almaguer, D.; Jaime-Pérez, J.C.; Mancías-Guerra, C. The effect of intra-articular injection of autologous bone marrow stem cells on pain and knee function in patients with osteoarthritis. Int. J. Rheum. Dis. 2018, 21, 140–147.
- Bensaïd, W.; Triffitt, J.T.; Blanchat, C.; Oudina, K.; Sedel, L.; Petite, H. A biodegradable fibrin scaffold for mesenchymal stem cell transplantation. Biomaterials 2003, 24, 2497–2502.
- Deng, J.; She, R.; Huang, W.; Dong, Z.; Mo, G.; Liu, B. A silk fibroin/chitosan scaffold in combination with bone marrow-derived mesenchymal stem cells to repair cartilage defects in the rabbit knee. J. Mater. Sci. Mater. Med. 2013, 24, 2037–2046.
- Cui, L.; Wu, Y.; Cen, L.; Zhou, H.; Yin, S.; Liu, G.; Liu, W.; Cao, Y. Repair of articular cartilage defect in non-weight bearing areas using adipose derived stem cells loaded polyglycolic acid mesh. Biomaterials 2009, 30, 2683–2693.
- Varghese, S.; Hwang, N.S.; Canver, A.C.; Theprungsirikul, P.; Lin, D.W.; Elisseeff, J. Chondroitin sulfate based niches for chondrogenic differentiation of mesenchymal stem cells. Matrix Biol. J. Int. Soc. Matrix Biol. 2008, 27, 12–21.
- Pittenger, M.F.; Mackay, A.M.; Beck, S.C.; Jaiswal, R.K.; Douglas, R.; Mosca, J.D.; Moorman, M.A.; Simonetti, D.W.; Craig, S.; Marshak, D.R. Multilineage potential of adult human mesenchymal stem cells. Science 1999, 284, 143–147.
- Lee, K.B.; Hui, J.H.; Song, I.C.; Ardany, L.; Lee, E.H. Injectable mesenchymal stem cell therapy for large cartilage defects—A porcine model. Stem Cells 2007, 25, 2964–2971.
- Kayakabe, M.; Tsutsumi, S.; Watanabe, H.; Kato, Y.; Takagishi, K. Transplantation of autologous rabbit BM-derived mesenchymal stromal cells embedded in hyaluronic acid gel sponge into osteochondral defects of the knee. Cytotherapy 2006, 8, 343–353.
- Lv, X.; He, J.; Zhang, X.; Luo, X.; He, N.; Sun, Z.; Xia, H.; Liu, V.; Zhang, L.; Lin, X.; et al. Comparative efficacy of autologous stromal vascular fraction and autologous adipose-derived mesenchymal stem cells combined with hyaluronic acid for the treatment of sheep osteoarthritis. Cell Transplant. 2018, 27, 1111–1125.
- Bartlett, W.; Skinner, J.A.; Gooding, C.R.; Carrington, R.W.; Flanagan, A.M.; Briggs, T.W.; Bentley, G. Autologous chondrocyte implantation versus matrix-induced autologous chondrocyte implantation for osteochondral defects of the knee: A prospective, randomised study. J. Bone Jt. Surg. Br. Vol. 2005, 87, 640–645.
- Davies, R.L.; Kuiper, N.J. Regenerative medicine: A review of the evolution of autologous chondrocyte implantation (ACI) therapy. Bioengineering 2019, 6, 22.
- Park, Y.B.; Ha, C.W.; Lee, C.H.; Yoon, Y.C.; Park, Y.G. Cartilage Regeneration in Osteoarthritic Patients by a Composite of Allogeneic Umbilical Cord Blood-Derived Mesenchymal Stem Cells and Hyaluronate Hydrogel: Results from a Clinical Trial for Safety and Proof-of-Concept with 7 Years of Extended Follow-Up. Stem Cells Transl. Med. 2017, 6, 613–621.
- Somoza, R.A.; Correa, D.; Labat, I.; Sternberg, H.; Forrest, M.E.; Khalil, A.M.; West, M.D.; Tesar, P.; Caplan, A.I. Transcriptome-wide analyses of human neonatal articular cartilage and human mesenchymal stem cell-derived cartilage provide a new molecular target for evaluating engineered cartilage. Tissue Eng. Part A 2018, 24, 335–350.
- Somoza, R.A.; Welter, J.F.; Correa, D.; Caplan, A.I. Chondrogenic differentiation of mesenchymal stem cells: Challenges and unfulfilled expectations. Tissue Eng. Part B Rev. 2014, 20, 596–608.
- Strioga, M.; Viswanathan, S.; Darinskas, A.; Slaby, O.; Michalek, J. Same or not the same? Comparison of adipose tissue-derived versus bone marrow-derived mesenchymal stem and stromal cells. Stem Cells Dev. 2012, 21, 2724–2752.
More
