Protein phosphorylation is a fundamental mechanism for many intracellular processes underlying cell life. This reversible mechanism, which is triggered by intra- and extra-cellular signals, regulates metabolism, transcription, proliferation, differentiation, cell movements, and apoptosis in countless cellular functions. Protein kinases form an enzyme family that catalyzes the transfer of the gamma-phosphate of adenosine triphosphate (ATP) to specific hydroxyl amino acids in protein substrates. On the other hand, protein phosphatases regulate the action of kinases, playing the role of regulators in the phosphorylation processes. The present investigation summarize the current knowledge on the roles playd by phosphatases in Chronic Myeloid Leukemia (CML).
- phosphatases
- kinases
- CML
- leukemia
- stem cell
1. Introduction
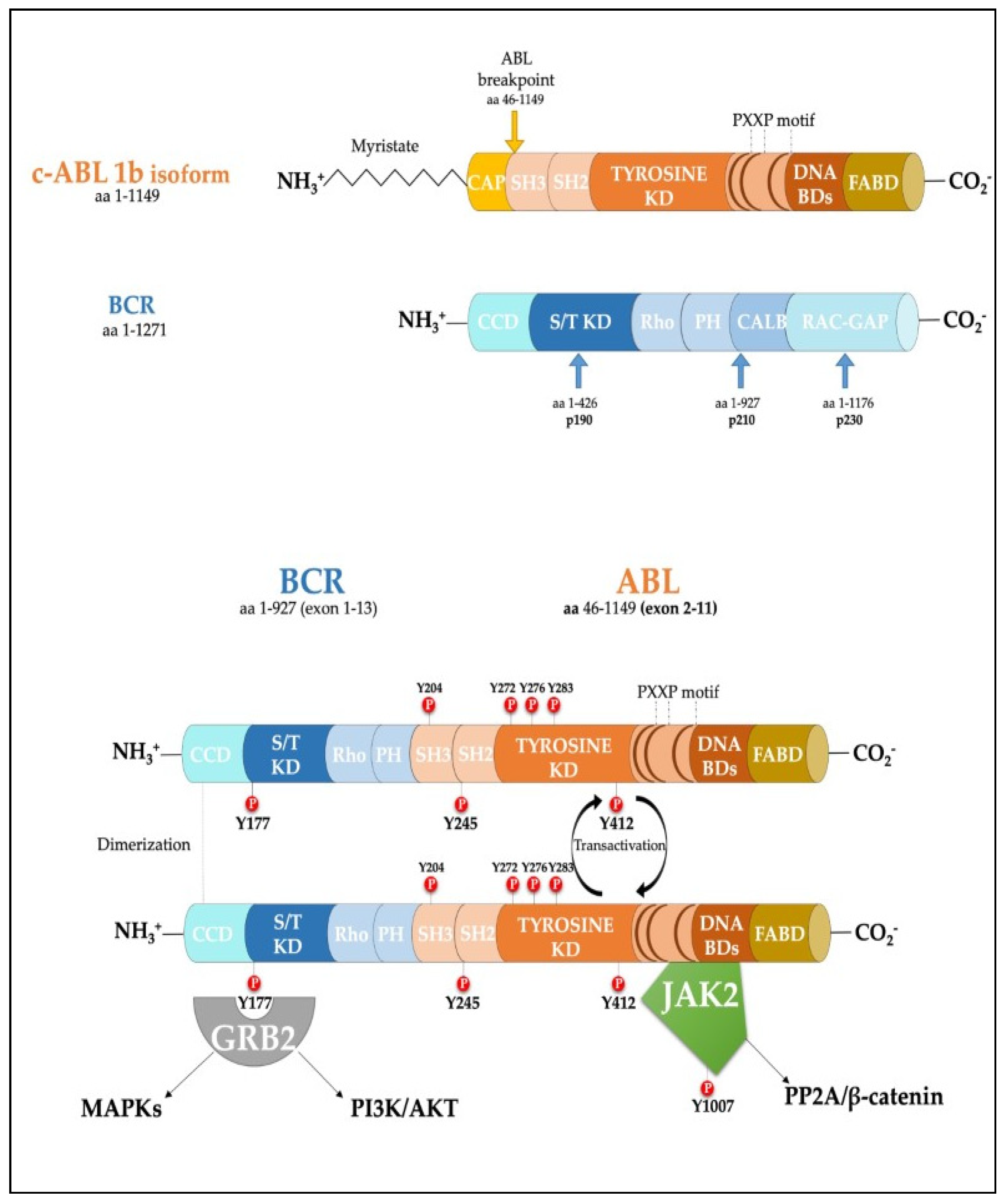 Figure 1. Scheme of the protein products derived from the BCR, ABL1b genes and the fusion product of the BCR-ABL1 oncogene. Major tyrosine phosphorylation sites are represented together with two well characterized interactors relevant for CML disease. SH Src homology domain; PTB Phosphotyrosine-binding domains; KD Kinase Domain; PH Pleckstrin homology domain; BD Binding Domain; FABD f-actin binding domain; CCD Coiled-coil domain.
Figure 1. Scheme of the protein products derived from the BCR, ABL1b genes and the fusion product of the BCR-ABL1 oncogene. Major tyrosine phosphorylation sites are represented together with two well characterized interactors relevant for CML disease. SH Src homology domain; PTB Phosphotyrosine-binding domains; KD Kinase Domain; PH Pleckstrin homology domain; BD Binding Domain; FABD f-actin binding domain; CCD Coiled-coil domain.2. Role of Phosphatases in the Regulation of Cell Proliferation
In the last three decades, the role of the BCR-ABL1 protein has been extensively analyzed and we have summarized the main pathways involved in Figure 2. One of the major signaling cascades that is altered involves the RAS mitogen-activated protein kinase family (MAPKs) [19], such as ERK1/2, MEK, JNK, and p38 families, which lead to insensitivity to growth factor stimuli and regulates the proliferative fate of cells [20][21]. In the active conformation, BCR-ABL1 possesses several tyrosine sites, such as Y245 in the SH2-kinase linker and Y412 in the activation loop, which are known to regulate function/activity (Figure 1). The human UBASH3B gene encodes for STS-1 phosphatase (suppressor of T-cell receptor signaling 1), the high expression of which has been correlated with the p190BCR-ABL1 form in acute lymphoblastic leukemia (ALL) patients’ samples [22], but is usually considered a BCR-ABL1 interactor [23][24]. In particular, other than being involved in signaling by other kinases, such as PDGFR, ZAP-70, and SYK, it can bind to the SH2–SH3 compartment of BCR-ABL1, producing strong dephosphorylation in the tyrosine sites of the ABL-portion, thus limiting its kinase activity. Remarkably, a CML-like disease mouse model with Sts-1/Sts-2 double knockout exacerbated the classical CML parameters and reduced mice survival, providing evidence supporting the leukemogenic role of Sts-1 [25]. The SH2-domain containing protein growth factor receptor bound protein-2 (GRB2) recognizes another fundamental Y177 binding site on the BCR domain which synergistically promotes CML, supporting RAS activation through Son of Sevenless (SOS) a guanine nucleotide exchange factor protein, and the scaffold adapter GAB2 (GRB2-associated binding protein 2) [26]. BCR-ABL1+ cells harboring a Y177F or SH2-domain mutation on GRB2 have exhibited less leukemogenic transformation and reduced proliferation by MAPK pathway disruption [27][28]. A recent study has also suggested that Y177 BCR-ABL1 phosphorylation is also regulated by ERα36-expression, an alternatively spliced variant of estrogen receptor α66 (ERα66), which is abnormally localized in the cytoplasm and cell membrane of BCR-ABL1+ cells. Synthetic ERα36 inhibitors prevented GRB2 from binding to BCR-ABL1, generation reduction in the downstream RAS/MAPKs pathway and cell proliferation impairment [29]. The major SHP2-binding protein in hematopoietic cells, GAB2, is part of the GRB2/GAB2 complex recruited on phosphorylated Y177. GAB2 is essential for myeloid and lymphoid leukemogenesis induced by BCR-ABL1, as demonstrated by the failure to develop a CML-like disease in a mouse model transplanted with BCR-ABL1 in GAB2−/− marrow cells. Its tyrosine phosphorylation in BCR-ABL1+ cells generates a docking site for signal cascade proteins, such as PI3K sub-unit p85α and SHP2 [30]; moreover, it induces the signal events, together with RAS activation, that lead to an increase of ERK function [27]. Downstream proliferative effects of the MAPKs pathway include transcription factors (TFs) such as NF-κB, CREB, ETS-1, AP-1, and cMYC, leading to cell cycle progression (involving CDKs) and anti-apoptotic mechanisms (involving BCL-2) [31]. SHP2 (PTPN11) plays a critical role in cell development due to its ability to support RAS/MAPK signaling, in response to numerous growth factors [32][33]. In the hematopoietic compartment, it is considered an oncogene as point mutations in its N-terminal SH2 inhibitory domain trigger the development of leukemia in different lineages [34]. An early study performed in CML CD34+ cells reported that the cytokine-independent colony formation capacity was altered by PTPN11-knockdown [35]. In particular, both mRNA and phosphoprotein levels appear to be greater in myeloid leukemia cells, addressing its presence/activity with a more hyper-proliferative phenotype [36]. Further evidence has confirmed SHP2 is required to initiate and maintain BCR-ABL1-mediated transformation, as GAB2 mutation in SH2 domain cannot bind SHP2 and, as a result, reduce myeloid and lymphoid leukemic burden. In line with this observation, various studies have described p-ERK activity reduction in BCR-ABL1 expressing myeloid cells lacking SHP2 phosphatase, thus emphasizing its role as a positive regulator of the RAS/MEK/ERK1-2 pathway in BCR-ABL1 signaling [27][37][38][39]. Speculation on reduced cell viability in this case may be traced back to RAS activation which occurs through SOS, but which also requires active SHP2 phosphatase. SHP2 can dephosphorylate and inactivate the p120 RAS-GAP protein (RAS GTPase-activating protein) by blocking its antagonistic action on RAS [33][40]. Although there are also implications for p-AKT-reduction, it seems to be mostly associated with GAB2 adapter protein impairment. On the other hand, Y177-GAB2 interaction is required to enforce another key player in leukemic transformation: Phosphoinositide-3 kinase (PI3K) [27] (see Figure 2).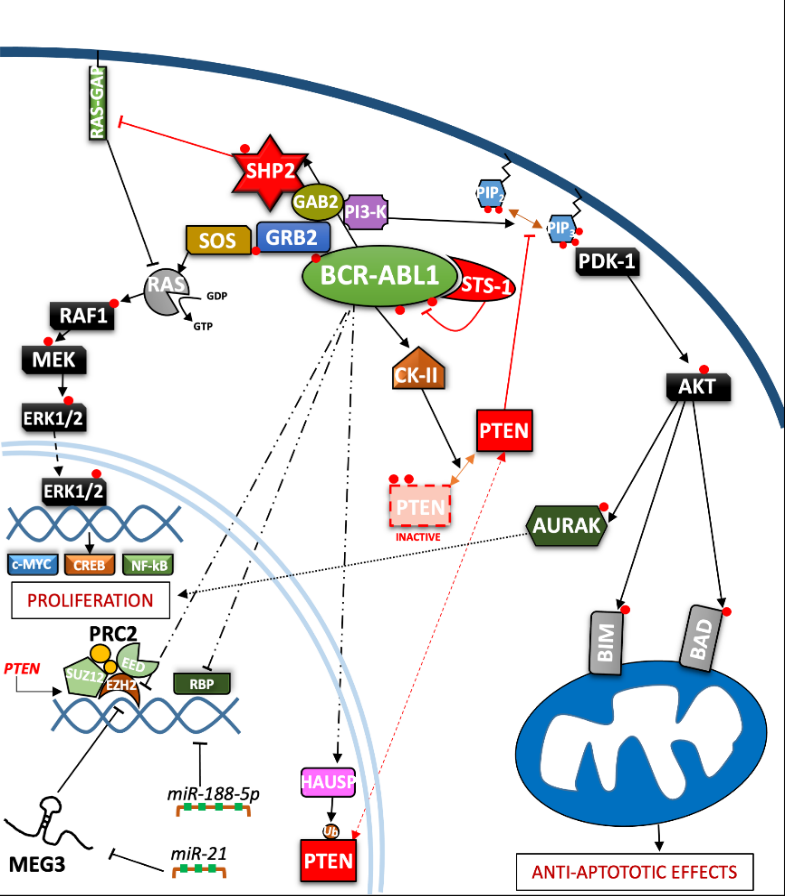
Figure 2. Depiction of the BCR-ABL1 kinase-driven proliferative processes in the context of CML. ERK = Extracellular signal-regulated kinases; MEG3 = Maternally expressed gene 3; PRC2 = Polycomb repressive complex 2; EZH = Enhancer of zeste polycomb repressive complex sub-unit.
The PI3K/AKT signal pathway plays a crucial role in a great variety of cancers, due to its strong relation with membrane tyrosine kinase receptors which are activated by multiple extracellular stimuli (cytokines, growth factors, etc.) [41]. Reportedly, the PI3K lipid kinase consists of p85 (regulatory) and p110 (catalytic) sub-units in a heterodimeric structure that is involved in inositol lipids (PtdIns(3,4)P2) phosphorylation, usually at the inner leaflet of the cytoplasmic membrane, initiating and controlling multiple cellular functions [42]. Specifically, PtdIns(3,4,5)P3 provides an anchor point for pleckstrin homology (PH) domain-containing proteins, such as activated protein kinase (AKT) and phosphoinositide-dependent protein kinase-1 (PDK-1). AKT is a serine/threonine kinase, belonging to the AGC family kinases, which is expressed in two isoforms in hematopoietic stem cells [43]. Activated p-AKT following by BCR-ABL1-activated PI3K, is able to inhibit the apoptotic process and support cell proliferation [44][45]. Skorski et al. reported the first direct data on PI3K (both sub-units) involvement in CML cell transformation [46], demonstrating how its downstream effector, AKT, was a critical growth regulatory switch in BCR-ABL1-expressing cells [47]. BCR-ABL1 can directly up-regulate AURK-A and AURK-B (serine/threonine kinases belonging to the Aurora family) through (at least in part) AKT, emphasizing a further pathway for cell, proliferation [48]. The PI3K pathway is constitutively activated in CML progenitor cells, as has been demonstrated by the elevated PtdIns(3,4,5)P3 levels found in CML progenitor cells, compared to their normal counterpart. Normal levels of PtdIns(3,4,5)P3 were restored, along with their capacity to respond near-normally to cytokine stimulation, by Imatinib treatment [49]. Many studies on this signaling pathway have been deepened in CML biology, thanks to the development of specific inhibitors to curb a wide range of effectors acting on this leukemia [50][51]. Notoriously, phosphatase and tensin homologue (PTEN) was the first tumor suppressor gene found to have a double specific phosphatase activity, acting on serine/threonine and tyrosine residues and the major antagonist of PI3K signaling, operating as a tumor suppressor in numerous solid neoplasms and leukemia [52][53]. By exploring the function of PTEN in BCR-ABL–expressing Ba/F3 cells, it has been revealed how BCR-ABL1 may induce PTEN phosphatase downregulation and a reduced p53 protein expression, regaining expression of both targets after TKI treatment [54][55]. An explanation was found in Pten gene promoter, where p53 can bind and promote its expression. Additionally, BCR-ABL1-expressing LSK cells sorted from CML mice, confirm a reduction of Pten mRNA, which also correlates with the downregulation of p53. Therefore, reduced disease progression related to PTEN expression could involve the reduction of CML cell proliferation through cell cycle arrest [54]. The nuclear-cytoplasmic shuttling enables PTEN to play its proper tumor suppressive function [56]. The mono-ubiquitinated PTEN form is predominantly nuclear, where it has been shown to play a tumor-suppressive role through a proliferation control mechanism. Herpesvirus ubiquitin-specific protease (HAUSP) is a critical modulator of protein ubiquitination, possibly regulating PTEN cytosolic partitioning [57]. In CML, BCR-ABL1 might phosphorylate HAUSP, triggering PTEN nuclear exclusion and causing proliferative advantages. Therefore, HAUSP together with nuclear PTEN depict a pivotal pathway in BCR-ABL1-induced proliferation [58]. In this way, Morotti et al. showed that PTEN-tail phosphorylation, mediated by BCR-ABL1-activated casein kinase II (CKII), caused a reduction of PTEN phosphatase activity, thus uncovering another inhibition mechanism present in CML [59]. Many different mechanisms might regulate PTEN expression in leukemic cells, including epigenetic shutdown, genomic loss, transcriptional repression, post-transcriptional regulation by lncRNA or microRNAs, etc. Polycomb repressive complex 2 (PRC2) contains two methyltransferase sub-units called enhancers of zeste 2 polycomb repressive complex subunit 1 and 2 (EZH1 and EZH2) that can modulate chromatin conformation by adding methyl groups on histone 3 (H3K27me2/3) [60]. EZH2 expression has been found to be upregulated in all three-phases of the disease in CML LSCs and, in particular, compared to other PRC2 components, it appears to be BCR-ABL1-kinase activity-dependent; meanwhile, EZH1 did not follow this trend, but was found to be downregulated [61][62]. Proliferation, self-renewal and viability of CML cells were drastically impeded by pharmacological interference with EZH2 activity (GSK126 inhibitor), or by reducing its expression through shRNA or in Ezh2−/− CML mice, resulting in increased survival in retroviral BCR-ABL1-transduced mouse models [61][63]. In addition, EZH2 knockdown decreased H3K27me3 levels on PTEN gene promoter and resulted in a significant increase in PTEN mRNA and protein expression in three human Ph+ cell lines, as well as in LSK (Lin−/Sca-1+/c-Kit+) cells from CML mice. Zhou et al. demonstrated the EZH2 inhibition-mediated beneficial effects on leukemia cells and prolonged survival of CML mice were compromised by the concurrent transduction of shRNA targeting PTEN [63]. Modification of PTEN expression might occur through maternally expressed gene 3 (MEG3), a long non-coding RNA (lncRNAs) associated with many cancers which has already been shown to regulate IM resistance in CML [64]. Lower MEG3 levels have been found in advanced stages of leukemia while, to the contrary, miR-21 was found to be over-expressed which was able to interact with MEG3 by decreasing its expression. The data indicated that MEG3 binding could modify the expression of MDM2 and EZH2 mRNA levels, producing DNA (cytosine-5)-methyltransferase 1 (DNMT1) protein upregulation and PTEN protein downregulation. Therefore, a reduced expression of miR-21 blocked the proliferation and promoted apoptosis of CML cells [65]. The upregulation of miR-188-5p represents another post-transcriptional regulatory mechanism for PTEN expression exhibited by CML cells. Indeed, miR-188-5p may directly target PTEN 3′-UTR, thus repressing it. Zi-Yuan Nie et al. demonstrated how the flavonoid Morin, isolated from Moraceae, might inhibit proliferation and induce apoptosis by repressing miR-188-5p expression, leading to PTEN/AKT pathway inhibition in CML cells both in the K562 cell line and mouse xenograft models [44]. Yin et al. obtained relevant data on other epigenetic modifications produced by the RBP2 protein. In particular, the BCR-ABL1/RBP2/PTEN pathway represents a feedback loop which is thought to increase proliferation, leading to blast phase (BP) transition. BCR-ABL1 activity directly inhibits directly RBP2 protein expression, resulting in the inhibition of PTEN transcription. The PTP domain of PTEN shares similar features with PTP1B phosphatase, presenting the same ability to bind and limit BCR-ABL1 phosphorylation [66] (Table 1).| Protein | Coding Gene | Role in CML | References |
|---|---|---|---|
| Data verified in primary CML cells or in leukemia mouse models | |||
| DUSP1 | DUSP1 | Implicated in TKI-response | [67] |
| STS-1 | UBASH3B | Decreases cell proliferation Direct BCR-ABL1 regulation |
[25][23] |
| FAP-1 | PTPN13 | Regulation of β-catenin functions Decreases TKI sensitivity |
[68][69][70][71][72][73] |
| PTPRG | PTPRG | Regulation of β-catenin functions Implicated in TKI response |
[74][75][76][77][78][79][80][81][82] |
| SHP1 | PTPN6 | Acts through the PP2A on BCR-ABL1 Regulates BCR-ABL1-independent IM resistance |
[39][83][84][85][86][87] |
| SHP2 | PTPN11 | Increases cell proliferation Implicated in TKI resistance |
[30][32][33][34][35][36][37][38][39] |
| PP2A | PPP2CA | Quiescence and Self-renewal regulation Governs TKI-response |
[83][84][88][89][90][91][80][92][93][94][87][95][96][97][98] |
| PTEN | PTEN | Control of cell proliferation | [44][54][55][56][57][58][59][61][63][64][66] |
| Data obtained only in CML cell lines | |||
| PTP1B | PTPN1 | Reduces cell viability Correlated with IM response |
[99][100][101][102][103][104] |
| LAR –LIPRIN Α1 | PPFIA1 | Mitigates BCR-ABL1 leukemogenesis | [105] |
| LYP | PTPN22 | Decreases IM sensitivity | [106] |
| LMW-PTP | ACP1 | Regulates autophagy process Correlated with IM resistance |
[107][108] |
| PP1Α | PPP1CA | Improves cell survival and apoptosis resistance | [109] |
| TC45/TC48 | TC-PTP | Implicated in IM- and INFα-resistance Regulation of proliferation and apoptosis |
[110][111][112] |
References
- Dan R Robinson; Yi-Mi Wu; Su-Fang Lin; The protein tyrosine kinase family of the human genome. Oncogene 2000, 19, 5548-5557, 10.1038/sj.onc.1203957.
- Mark A. Lemmon; Joseph Schlessinger; Cell Signaling by Receptor Tyrosine Kinases. Cell 2010, 141, 1117-1134, 10.1016/j.cell.2010.06.011.
- Neel H. Shah; Jeanine F. Amacher; Laura M. Nocka; John Kuriyan; The Src module: an ancient scaffold in the evolution of cytoplasmic tyrosine kinases. Critical Reviews in Biochemistry and Molecular Biology 2018, 53, 535-563, 10.1080/10409238.2018.1495173.
- Sheila M. Thomas; Joan S. Brugge; CELLULAR FUNCTIONS REGULATED BY SRC FAMILY KINASES. Annual Review of Cell and Developmental Biology 1997, 13, 513-609, 10.1146/annurev.cellbio.13.1.513.
- Robert Roskoski; A historical overview of protein kinases and their targeted small molecule inhibitors. Pharmacological Research 2015, 100, 1-23, 10.1016/j.phrs.2015.07.010.
- Peter Blume-Jensen; Tony Hunter; Oncogenic kinase signalling. Nature 2001, 411, 355-365, 10.1038/35077225.
- Bhushan Nagar; Oliver Hantschel; Markus Seeliger; Jason M. Davies; William I. Weis; Giulio Superti-Furga; John Kuriyan; Organization of the SH3-SH2 Unit in Active and Inactive Forms of the c-Abl Tyrosine Kinase. Molecular Cell 2006, 21, 787-798, 10.1016/j.molcel.2006.01.035.
- Karel Dorey; John R Engen; Jana Kretzschmar; Matthias Wilm; Gitte Neubauer; Thomas Schindler; Giulio Superti-Furga; Phosphorylation and structure-based functional studies reveal a positive and a negative role for the activation loop of the c-Abl tyrosine kinase. Oncogene 2001, 20, 8075-8084, 10.1038/sj.onc.1205017.
- J R McWhirter; D L Galasso; J Y Wang; A coiled-coil oligomerization domain of Bcr is essential for the transforming function of Bcr-Abl oncoproteins.. Molecular and Cellular Biology 1993, 13, 7587-7595, 10.1128/mcb.13.12.7587.
- G Q Daley; R A Van Etten; D Baltimore; Induction of chronic myelogenous leukemia in mice by the P210bcr/abl gene of the Philadelphia chromosome. Science 1990, 247, 824-830, 10.1126/science.2406902.
- Brian J. Druker; Translation of the Philadelphia chromosome into therapy for CML. Blood 2008, 112, 4808-4817, 10.1182/blood-2008-07-077958.
- Jane F Apperley; Part I: Mechanisms of resistance to imatinib in chronic myeloid leukaemia. The Lancet Oncology 2007, 8, 1018-1029, 10.1016/s1470-2045(07)70342-x.
- Susan Branford; Zbigniew Rudzki; Sonya Walsh; Ian Parkinson; Andrew Grigg; Jeff Szer; Kerry Taylor; Richard Herrmann; John F. Seymour; Chris Arthur; et al.David JoskeKevin LynchTim Hughes Detection of BCR-ABL mutations in patients with CML treated with imatinib is virtually always accompanied by clinical resistance, and mutations in the ATP phosphate-binding loop (P-loop) are associated with a poor prognosis. Blood 2003, 102, 276-283, 10.1182/blood-2002-09-2896.
- Jorge Cortes; Elias Jabbour; Hagop Kantarjian; C. Cameron Yin; Jianqin Shan; Susan O'brien; Guillermo Garcia-Manero; Francis Giles; Megan Breeden; Nubia Reeves; et al.William G. WierdaDan Jones Dynamics of BCR-ABL kinase domain mutations in chronic myeloid leukemia after sequential treatment with multiple tyrosine kinase inhibitors. Blood 2007, 110, 4005-4011, 10.1182/blood-2007-03-080838.
- T P Hughes; G Saglio; A Quintás-Cardama; M J Mauro; D-W Kim; J H Lipton; M B Bradley-Garelik; J Ukropec; A Hochhaus; BCR-ABL1 mutation development during first-line treatment with dasatinib or imatinib for chronic myeloid leukemia in chronic phase. Leukemia 2015, 29, 1832-1838, 10.1038/leu.2015.168.
- David P. Labbé; Serge Hardy; Michel L. Tremblay; Protein Tyrosine Phosphatases in Cancer. Progress in Molecular Biology and Translational Science 2012, 2, 253-306, 10.1016/b978-0-12-396456-4.00009-2.
- Angela Bononi; Chiara Agnoletto; Elena De Marchi; Saverio Marchi; Simone Patergnani; Massimo Bonora; Carlotta Giorgi; Sonia Missiroli; Federica Poletti; Alessandro Rimessi; et al.Paolo Pinton Protein Kinases and Phosphatases in the Control of Cell Fate. Enzyme Research 2011, 2011, 1-26, 10.4061/2011/329098.
- Peter P. Ruvolo; Role of protein phosphatases in the cancer microenvironment. Biochimica et Biophysica Acta 2019, 1866, 144-152, 10.1016/j.bbamcr.2018.07.006.
- Mario Notari; Paolo Neviani; Ramasamy Santhanam; Bradley W. Blaser; Ji-Suk Chang; Annamaria Galietta; Anne E. Willis; Denis C. Roy; Michael A. Caligiuri; Guido Marcucci; et al.Danilo Perrotti A MAPK/HNRPK pathway controls BCR/ABL oncogenic potential by regulating MYC mRNA translation. Blood 2006, 107, 2507-2516, 10.1182/blood-2005-09-3732.
- Leonidas C. Platanias; Map kinase signaling pathways and hematologic malignancies. Blood 2003, 101, 4667-4679, 10.1182/blood-2002-12-3647.
- Chi-Dug Kang; Seok-Dong Yoo; Byung-Wook Hwang; Kwang-Woon Kim; Dong-Wan Kim; Cheol-Min Kim; Sun-Hee Kim; Byung-Seon Chung; The inhibition of ERK/MAPK not the activation of JNK/SAPK is primarily required to induce apoptosis in chronic myelogenous leukemic K562 cells. Leukemia Research 2000, 24, 527-534, 10.1016/s0145-2126(00)00010-2.
- Dejan Juric; Norman J. Lacayo; Meghan C. Ramsey; Janis Racevskis; Peter H. Wiernik; Jacob M. Rowe; Anthony H. Goldstone; Peter J. O'dwyer; Elisabeth Paietta; Branimir I. Sikic; et al. Differential Gene Expression Patterns and Interaction Networks in BCR-ABL–Positive and –Negative Adult Acute Lymphoblastic Leukemias. Journal of Clinical Oncology 2007, 25, 1341-1349, 10.1200/jco.2006.09.3534.
- J A Cutler; R Tahir; S K Sreenivasamurthy; C Mitchell; S Renuse; R S Nirujogi; A H Patil; M Heydarian; X Wong; X Wu; et al.Tai-Chung HuangMin-Sik KimK L ReddyA Pandey Differential signaling through p190 and p210 BCR-ABL fusion proteins revealed by interactome and phosphoproteome analysis. Leukemia 2017, 31, 1513-1524, 10.1038/leu.2017.61.
- Marc Brehme; Oliver Hantschel; Jacques Colinge; Ines Kaupe; Melanie Planyavsky; Thomas Köcher; Karl Mechtler; Keiryn L. Bennett; Giulio Superti-Furga; Charting the molecular network of the drug target Bcr-Abl. Proceedings of the National Academy of Sciences 2009, 106, 7414-7419, 10.1073/pnas.0900653106.
- Afsar A. Mian; Ines Baumann; Marcus Liebermann; Florian Grebien; Giulio Superti-Furga; Martin Ruthardt; Oliver G. Ottmann; Oliver Hantschel; The phosphatase UBASH3B/Sts-1 is a negative regulator of Bcr-Abl kinase activity and leukemogenesis. Leukemia 2019, 33, 2319-2323, 10.1038/s41375-019-0468-y.
- Ann Marie Pendergast; Lawrence A. Quilliam; Larry D. Cripe; Craig H. Bassing; Zonghan Dai; Nanxin Li; Andreas Batzer; Kelly M. Rabun; Channing J. Der; Joseph Schlessinger; et al.Mikhail L. Gishizky BCR-ABL-induced oncogenesis is mediated by direct interaction with the SH2 domain of the GRB-2 adaptor protein. Cell 1993, 75, 175-185, 10.1016/s0092-8674(05)80094-7.
- Martin Sattler; M.Golam Mohi; Yuri B Pride; Laura R Quinnan; Nicole A Malouf; Klaus Podar; Franck Gesbert; Hiromi Iwasaki; Shaoguang Li; Richard A Van Etten; et al.Haihua GuJames D GriffinBenjamin G Neel Critical role for Gab2 in transformation by BCR/ABL. Cancer Cell 2002, 1, 479-492, 10.1016/s1535-6108(02)00074-0.
- H Modi; L Li; S Chu; J Rossi; J-K Yee; R Bhatia; Inhibition of Grb2 expression demonstrates an important role in BCR–ABL-mediated MAPK activation and transformation of primary human hematopoietic cells. Leukemia 2010, 25, 305-312, 10.1038/leu.2010.257.
- Min Chen; Ali G. Turhan; Hongxia Ding; Qingcong Lin; Kun Meng; Xiaoyan Jiang; Targeting BCR-ABL+ stem/progenitor cells and BCR-ABL-T315I mutant cells by effective inhibition of the BCR-ABL-Tyr177-GRB2 complex. Oncotarget 2017, 8, 43662-43677, 10.18632/oncotarget.18216.
- Haihua Gu; Joanne C. Pratt; Steven J. Burakoff; Benjamin G. Neel; Cloning of p97/Gab2, the Major SHP2-Binding Protein in Hematopoietic Cells, Reveals a Novel Pathway for Cytokine-Induced Gene Activation. Molecular Cell 1998, 2, 729-740, 10.1016/s1097-2765(00)80288-9.
- Y. Zhang; Ernesto Diaz-Flores; Geqiang Li; Zhengqi Wang; Zizhen Kang; Eleonora Haviernikova; Sara Rowe; Cheng-Kui Qu; William Tse; Kevin M. Shannon; et al.Kevin D. Bunting Abnormal hematopoiesis in Gab2 mutant mice. Blood 2007, 110, 116-124, 10.1182/blood-2006-11-060707.
- Cheng-Kui Qu; Suzanne Nguyen; Jianzhu Chen; Gen-Sheng Feng; Requirement of Shp-2 tyrosine phosphatase in lymphoid and hematopoietic cell development. Blood 2001, 97, 911-914, 10.1182/blood.v97.4.911.
- Marie Dance; Alexandra Montagner; Jean-Pierre Salles; Armelle Yart; Patrick Raynal; The molecular functions of Shp2 in the Ras/Mitogen-activated protein kinase (ERK1/2) pathway. Cellular Signalling 2008, 20, 453-459, 10.1016/j.cellsig.2007.10.002.
- Ruchi Pandey; Mallika Saxena; Reuben Kapur; Role of SHP2 in hematopoiesis and leukemogenesis. Current Opinion in Hematology 2017, 24, 307-313, 10.1097/moh.0000000000000345.
- Michaela Scherr; Anuhar Chaturvedi; Karin Battmer; Iris Dallmann; Beate Schultheis; Arnold Ganser; Matthias Eder; Enhanced sensitivity to inhibition of SHP2, STAT5, and Gab2 expression in chronic myeloid leukemia (CML). Blood 2006, 107, 3279-3287, 10.1182/blood-2005-08-3087.
- Rongzhen Xu; Yingzi Yu; Shu Zheng; Xiaoying Zhao; Qinghua Dong; Zhiwen He; Yun Liang; Qinghua Lu; Yongmin Fang; Xiaoxian Gan; et al.Xiaohua XuSuzhan ZhangQi DongXiaohong ZhangGen-Sheng Feng Overexpression of Shp2 tyrosine phosphatase is implicated in leukemogenesis in adult human leukemia. Blood 2005, 106, 3142-3149, 10.1182/blood-2004-10-4057.
- Shengqing Gu; Wayne W. Chan; Golam Mohi; Joel Rosenbaum; Azin Sayad; Zhibin Lu; Carl Virtanen; Shaoguang Li; Benjamin G. Neel; Richard A. Van Etten; et al. Distinct GAB2 signaling pathways are essential for myeloid and lymphoid transformation and leukemogenesis by BCR-ABL1. Blood 2016, 127, 1803-1813, 10.1182/blood-2015-06-653006.
- Shengqing Gu; Azin Sayad; Gordon Chan; Wentian Yang; Zhibin Lu; Carl Virtanen; Richard A. Van Etten; Benjamin G. Neel; SHP2 is required for BCR-ABL1-induced hematologic neoplasia. Leukemia 2017, 32, 203-213, 10.1038/leu.2017.250.
- Nicola Esposito; Irene Colavita; Concetta Quintarelli; Agostino Rodeo Sica; Anna Lucia Peluso; Luigia Luciano; Marco Picardi; Luigi Del Vecchio; Tonia Buonomo; Timothy P. Hughes; et al.Deborah WhiteJerald P. RadichDomenico RussoSusan BranfordGiuseppe SaglioJunia V. MeloRosanna MartinelliMargherita RuoppoloThea KalebicGiovanni MartinelliFabrizio Pane SHP-1 expression accounts for resistance to imatinib treatment in Philadelphia chromosome–positive cells derived from patients with chronic myeloid leukemia. Blood 2011, 118, 3634-3644, 10.1182/blood-2011-03-341073.
- Alexandra Montagner; Armelle Yart; Marie Dance; Bertrand Perret; Jean-Pierre Salles; Patrick Raynal; A Novel Role for Gab1 and SHP2 in Epidermal Growth Factor-induced Ras Activation. Journal of Biological Chemistry 2005, 280, 5350-5360, 10.1074/jbc.m410012200.
- Michael G. Kharas; David A. Fruman; ABL Oncogenes and Phosphoinositide 3-Kinase: Mechanism of Activation and Downstream Effectors. Cancer Research 2005, 65, 2047-2053, 10.1158/0008-5472.can-04-3888.
- Sarah K. Tasian; David T. Teachey; Susan R. Rheingold; Targeting the PI3K/mTOR Pathway in Pediatric Hematologic Malignancies. Frontiers in Oncology 2014, 4, 108, 10.3389/fonc.2014.00108.
- Marisa Juntilla; Vineet D. Patil; Marco Calamito; Rohan P. Joshi; Morris J. Birnbaum; Gary A. Koretzky; AKT1 and AKT2 maintain hematopoietic stem cell function by regulating reactive oxygen species. Blood 2010, 115, 4030-4038, 10.1182/blood-2009-09-241000.
- Zi-Yuan Nie; Lin Yang; Xiao-Jun Liu; Zhan Yang; Gao-Shan Yang; Jing Zhou; Yan Qin; Jing Yu; Ling-Ling Jiang; Jin-Kun Wen; et al.Jian-Min Luo Morin Inhibits Proliferation and Induces Apoptosis by Modulating the miR-188-5p/PTEN/AKT Regulatory Pathway in CML Cells. Molecular Cancer Therapeutics 2019, 18, 2296-2307, 10.1158/1535-7163.mct-19-0051.
- Yu Chen; Tongtong Wang; Jing Du; Yanchun Li; Xin Wang; Yi Zhou; Xingxing Yu; Weimin Fan; Qiaojuan Zhu; Xiangmin Tong; et al.Ying Wang The Critical Role of PTEN/PI3K/AKT Signaling Pathway in Shikonin-Induced Apoptosis and Proliferation Inhibition of Chronic Myeloid Leukemia. Cellular Physiology and Biochemistry 2018, 47, 981-993, 10.1159/000490142.
- T Skorski; P Kanakaraj; M Nieborowska-Skorska; Mariusz Z. Ratajczak; Sc Wen; G Zon; Am Gewirtz; B Perussia; B Calabretta; Phosphatidylinositol-3 kinase activity is regulated by BCR/ABL and is required for the growth of Philadelphia chromosome-positive cells. Blood 1995, 86, 726-736, 10.1182/blood.v86.2.726.bloodjournal862726.
- Tomasz Skorski; Alfonso Bellacosa; Margaret Nieborowska‐Skorska; Miroslaw Majewski; Robert Martinez; John K. Choi; Rossana Trotta; Pawel Wlodarski; Danilo Perrotti; Tung O. Chan; et al.Mariusz A. WasikPhilip N. TsichlisBruno Calabretta Transformation of hematopoietic cells by BCR/ABL requires activation of a PI-3k/Akt-dependent pathway.. The EMBO Journal 1997, 16, 6151-6161, 10.1093/emboj/16.20.6151.
- Jing Yang; Takayuki Ikezoe; Chie Nishioka; Keiko Udaka; Akihito Yokoyama; Bcr‐Abl activates AURKA and AURKB in chronic myeloid leukemia cells via AKT signaling. International Journal of Cancer 2013, 134, 1183-1194, 10.1002/ijc.28434.
- H G Hamzah; A Pierce; W A Stewart; C Peter Downes; Alexander Gray; A Irvine; E Spooncer; A D Whetton; Chronic myeloid leukemia CD34+ cells have elevated levels of phosphatidylinositol 3,4,5 trisphosphate (PtdIns(3,4,5)P3) and lack a PtdIns(3,4,5)P3 response to cytokines and chemotactic factors; effects reversed by imatinib. Leukemia 2005, 19, 1851-1853, 10.1038/sj.leu.2403919.
- K Schuster; J Zheng; A A Arbini; C C Zhang; P P Scaglioni; Selective targeting of the mTORC1/2 protein kinase complexes leads to antileukemic effects in vitro and in vivo. Blood Cancer Journal 2011, 1, e34-e34, 10.1038/bcj.2011.30.
- Pengliang Xin; Chuntuan Li; Yan Zheng; Qunyi Peng; Huifang Xiao; Yuanling Huang; Xiongpeng Zhu; Efficacy of the dual PI3K and mTOR inhibitor NVP-BEZ235 in combination with imatinib mesylate against chronic myelogenous leukemia cell lines. Drug Design, Development and Therapy 2017, ume11, 1115-1126, 10.2147/dddt.s132092.
- Anthony J. Trimboli; Carmen Z. Cantemir-Stone; Fu Li; Julie A. Wallace; Anand Merchant; Nicholas Creasap; John C. Thompson; Enrico Caserta; Hui Wang; Jean-Leon Chong; et al.Shan NaiduGuo WeiSudarshana SharmaJulie A. StephensSoledad A. FernandezMetin N. GurcanMichael B. WeinsteinSanford H. BarskyLisa YeeThomas RosolPaul C. StrombergMichael L. RobinsonFrancois PepinMichael HallettMorag ParkMichael OstrowskiGustavo Leone Pten in stromal fibroblasts suppresses mammary epithelial tumours. Nature 2009, 461, 1084-1091, 10.1038/nature08486.
- C Nishioka; T Ikezoe; J Yang; A Yokoyama; Long-term exposure of leukemia cells to multi-targeted tyrosine kinase inhibitor induces activations of AKT, ERK and STAT5 signaling via epigenetic silencing of the PTEN gene. Leukemia 2010, 24, 1631-1640, 10.1038/leu.2010.145.
- Cong Peng; Yaoyu Chen; Zhongfa Yang; Haojian Zhang; Lori Osterby; Alan G. Rosmarin; Shaoguang Li; PTEN is a tumor suppressor in CML stem cells and BCR-ABL–induced leukemias in mice. Blood 2010, 115, 626-635, 10.1182/blood-2009-06-228130.
- Cristina Panuzzo; Sabrina Crivellaro; Giovanna Carra; Angelo Guerrasio; Giuseppe Saglio; Alessandro Morotti; BCR-ABL Promotes PTEN Downregulation in Chronic Myeloid Leukemia. PLOS ONE 2014, 9, e110682, 10.1371/journal.pone.0110682.
- C. Bassi; J. Ho; T. Srikumar; R. J. O. Dowling; C. Gorrini; S. J. Miller; T. W. Mak; B. G. Neel; B. Raught; Vuk Stambolic; et al. Nuclear PTEN Controls DNA Repair and Sensitivity to Genotoxic Stress. Science 2013, 341, 395-399, 10.1126/science.1236188.
- Min Sup Song; Leonardo Salmena; Arkaitz Carracedo; Ainara Egia; Francesco Lo-Coco; Julie Teruya-Feldstein; Pier Paolo Pandolfi; The deubiquitinylation and localization of PTEN are regulated by a HAUSP–PML network. Nature 2008, 455, 813-817, 10.1038/nature07290.
- A Morotti; C Panuzzo; S Crivellaro; B Pergolizzi; U Familiari; A H Berger; G Saglio; Pier Paolo Pandolfi; BCR-ABL disrupts PTEN nuclear-cytoplasmic shuttling through phosphorylation-dependent activation of HAUSP. Leukemia 2013, 28, 1326-1333, 10.1038/leu.2013.370.
- Alessandro Morotti; Cristina Panuzzo; Sabrina Crivellaro; Giovanna Carrà; Carmen Fava; Angelo Guerrasio; Pier Paolo Pandolfi; Giuseppe Saglio; BCR-ABL inactivates cytosolic PTEN through Casein Kinase II mediated tail phosphorylation. Cell Cycle 2015, 14, 973-979, 10.1080/15384101.2015.1006970.
- Raphaël Margueron; Danny Reinberg; The Polycomb complex PRC2 and its mark in life. Nature 2011, 469, 343-349, 10.1038/nature09784.
- Huafeng Xie; Cong Peng; Jialiang Huang; Bin E. Li; Woojin Kim; Elenoe C. Smith; Yuko Fujiwara; Jun Qi; Giulia Cheloni; Partha P. Das; et al.Minh NguyenShaoguang LiJames E. BradnerStuart H. Orkin Chronic Myelogenous Leukemia– Initiating Cells Require Polycomb Group Protein EZH2. Cancer Discovery 2016, 6, 1237-1247, 10.1158/2159-8290.cd-15-1439.
- Mary T. Scott; Koorosh Korfi; Peter Saffrey; Lisa E.M. Hopcroft; Ross Kinstrie; Francesca Pellicano; Carla Guenther; Paolo Gallipoli; Michelle Cruz; Karen Dunn; et al.Heather G. JorgensenJennifer E. CasselsAshley HamiltonAndrew CrossanAmy SinclairTessa L. HolyoakeDavid Vetrie Epigenetic Reprogramming Sensitizes CML Stem Cells to Combined EZH2 and Tyrosine Kinase Inhibition. Cancer Discovery 2016, 6, 1248-1257, 10.1158/2159-8290.cd-16-0263.
- Jingfeng Zhou; Danian Nie; Juan Li; Xin Du; Yuhong Lu; Yangqiu Li; Chang Liu; Wei Dai; Yun Wang; Yanli Jin; et al.Jingxuan Pan PTEN Is Fundamental for Elimination of Leukemia Stem Cells Mediated by GSK126 Targeting EZH2 in Chronic Myelogenous Leukemia. Clinical Cancer Research 2017, 24, 145-157, 10.1158/1078-0432.ccr-17-1533.
- Xiangyu Zhou; Ping Yuan; Qi Liu; Zhiqiang Liu; LncRNA MEG3 Regulates Imatinib Resistance in Chronic Myeloid Leukemia via Suppressing MicroRNA-21. Biomolecules & Therapeutics 2017, 25, 490-496, 10.4062/biomolther.2016.162.
- Ziye Li; Lin Yang; Xiaojun Liu; Ziyuan Nie; Jianmin Luo; Long noncoding RNA MEG3 inhibits proliferation of chronic myeloid leukemia cells by sponging microRNA21. Biomedicine & Pharmacotherapy 2018, 104, 181-192, 10.1016/j.biopha.2018.05.047.
- Xiaolin Yin; Minran Zhou; Yue Fu; Lin Yang; Man Xu; Ting Sun; XiaoMing Wang; Tao Huang; Chunyan Chen; Histone demethylase RBP2 mediates the blast crisis of chronic myeloid leukemia through an RBP2/PTEN/BCR-ABL cascade.. Cellular Signalling 2019, 63, 109360, 10.1016/j.cellsig.2019.109360.
- Kesarwani, M.; Kincaid, Z.; Gomaa, A.; Huber, E.; Rohrabaugh, S.; Siddiqui, Z.; Bouso, M.F.; Latif, T.; Xu, M.; Komurov, K.; et al. Targeting c-FOS and DUSP1 abrogates intrinsic resistance to tyrosine-kinase inhibitor therapy in BCR-ABL-induced leukemia. Nat. Med. 2017, 23, 472–482.
- Huang, W.; Zhu, C.; Wang, H.; Horvath, E.; Eklund, E.A. The Interferon Consensus Sequence-binding Protein (ICSBP/IRF8) Represses PTPN13 Gene Transcription in Differentiating Myeloid Cells. J. Biol. Chem. 2008, 283, 7921–7935.
- Hao, S.X.; Ren, R.; Jahn, T.; Seipel, P.; Urschel, S.; Peschel, C.; Duyster, J. Expression of Interferon Consensus Sequence Binding Protein (ICSBP) Is Downregulated in Bcr-Abl-Induced Murine Chronic Myelogenous Leukemia-Like Disease, and Forced Coexpression of ICSBP Inhibits Bcr-Abl-Induced Myeloproliferative Disorder. Mol. Cell. Biol. 2000, 20, 979–991.
- Schmidt, M.; Nagel, S.; Proba, J.; Thiede, C.; Ritter, M.; Waring, J.F.; Rosenbauer, F.; Huhn, D.; Wittig, B.; Horak, I.; et al. Lack of interferon consensus sequence binding protein (ICSBP) transcripts in human myeloid leukemias. Blood 1998, 91, 22–29.
- Huang, W.; Bei, L.; Eklund, E.A. Fas-associated phosphatase 1 (Fap1) influences betacatenin activity in myeloid progenitor cells expressing the Bcr-abl oncogene. J. Biol. Chem. 2013, 288, 12766–12776.
- Huang, W.; Bei, L.; Eklund, E.A. Fas-associated phosphatase 1 mediates Fas resistance in myeloid progenitor cells expressing the Bcr–abl oncogene. Leuk. Lymphoma 2012, 54, 619–630.
- Huang, W.; Luan, C.-H.; Hjort, E.E.; Bei, L.; Mishra, R.; Sakamoto, K.M.; Platanias, L.C.; Eklund, E.A. The role of Fas-associated phosphatase 1 in leukemia stem cell persistence during tyrosine kinase inhibitor treatment of chronic myeloid leukemia. Leukemia 2016, 30, 1502–1509.
- Lissandrini, D.; Vermi, W.; Vezzalini, M.; Sozzani, S.; Facchetti, F.; Bellone, G.; Mafficini, A.; Gentili, F.; Ennas, M.G.; Tecchio, C.; et al. Receptor-type protein tyrosine phosphatase gamma (PTPγ), a new identifier for myeloid dendritic cells and specialized macrophages. Blood 2006, 108, 4223–4231.
- Sorio, C.; Melotti, P.; D’Arcangelo, D.; Mendrola, J.; Calabretta, B.; Croce, C.M.; Huebner, K. Receptor protein tyrosine phosphatase gamma, Ptp gamma, regulates hematopoietic differentiation. Blood 1997, 90, 49–57.
- Mafficini, A.; Vezzalini, M.; Zamai, L.; Galeotti, L.; Bergamini, G.; Della Peruta, M.; Melotti, P.; Sorio, C. Protein Tyrosine Phosphatase Gamma (PTPgamma) is a Novel Leukocyte Marker Highly Expressed by CD34 Precursors. Biomark. Insights 2007, 2, 218–225.
- Della Peruta, M.; Martinelli, G.; Moratti, E.; Pintani, D.; Vezzalini, M.; Mafficini, A.; Grafone, T.; Iacobucci, I.; Soverini, S.; Murineddu, M.; et al. Protein tyrosine phosphatase receptor type is a functional tumor suppressor gene specifically downregulated in chronic myeloid leukemia. Cancer Res. 2010, 70, 8896–8906.
- Vezzalini, M.; Mafficini, A.; Tomasello, L.; Lorenzetto, E.; Moratti, E.; Fiorini, Z.; Holyoake, T.L.; Pellicano, F.; Krampera, M.; Tecchio, C.; et al. A new monoclonal antibody detects downregulation of protein tyrosine phosphatase receptor type gamma in chronic myeloid leukemia patients. J. Hematol. Oncol. 2017, 10, 129.
- Tomasello, L.; Vezzalini, M.; Boni, C.; Bonifacio, M.; Scaffidi, L.; Yassin, M.; Al-Dewik, N.; Takam Kamga, P.; Krampera, M.; Sorio, C. Regulative Loop between beta-catenin and Protein Tyrosine Receptor Type gamma in Chronic Myeloid Leukemia. Int. J. Mol. Sci. 2020, 21, 2298.
- Ismail, M.A.; Samara, M.; Al Sayab, A.; Alsharshani, M.; Yassin, M.A.; Varadharaj, G.; Vezzalini, M.; Tomasello, L.; Monne, M.; Morsi, H.; et al. Aberrant DNA methylation of PTPRG as one possible mechanism of its under-expression in CML patients in the State of Qatar. Mol. Genet. Genom. Med. 2020, 8, 1319.
- Ismail, M.A.; Vezzalini, M.; Morsi, H.; Abujaber, A.; Al Sayab, A.; Siveen, K.; Yassin, M.A.; Monne, M.; Samara, M.; Cook, R.; et al. Predictive value of tyrosine phosphatase receptor gamma for the response to treatment tyrosine kinase inhibitors in chronic myeloid leukemia patients. Sci. Rep. 2021, 11, 8833.
- Drube, J.; Ernst, T.; Pfirrmann, M.; Albert, B.V.; Drube, S.; Reich, D.; Kresinsky, A.; Halfter, K.; Sorio, C.; Fabisch, C.; et al. PTPRG and PTPRC modulate nilotinib response in chronic myeloid leukemia cells. Oncotarget 2018, 9, 9442–9455.
- Neviani, P.; Santhanam, R.; Trotta, R.; Notari, M.; Blaser, B.W.; Liu, S.; Mao, H.; Chang, J.S.; Galietta, A.; Uttam, A.; et al. The tumor suppressor PP2A is functionally inactivated in blast crisis CML through the inhibitory activity of the BCR/ABL-regulated SET protein. Cancer Cell 2005, 8, 355–368.
- Samanta, A.K.; Chakraborty, S.N.; Wang, Y.; Kantarjian, H.; Sun, X.; Hood, J.; Perrotti, D.; Arlinghaus, R.B. Jak2 inhibition deactivates Lyn kinase through the SET–PP2A–SHP1 pathway, causing apoptosis in drug-resistant cells from chronic myelogenous leukemia patients. Oncogene 2009, 28, 1669–1681.
- Li, Y.; Liu, X.; Guo, X.; Liu, X.; Luo, J. DNA methyltransferase 1 mediated aberrant methylation and silencing of SHP-1 gene in chronic myelogenous leukemia cells. Leuk. Res. 2017, 58, 9–13.
- Zhang, X.; Yang, L.; Liu, X.; Nie, Z.; Wang, X.; Pan, Y.; Luo, J. Research on the epigenetic regulation mechanism of thePTPN6gene in advanced chronic myeloid leukaemia. Br. J. Haematol. 2017, 178, 728–738.
- Salas, A.; Ponnusamy, S.; Senkal, C.E.; Meyers-Needham, M.; Selvam, S.P.; Saddoughi, S.A.; Apohan, E.; Sentelle, R.D.; Smith, C.; Gault, C.R.; et al. Sphingosine kinase-1 and sphingosine 1-phosphate receptor 2 mediate Bcr-Abl1 stability and drug resistance by modulation of protein phosphatase 2A. Blood 2011, 117, 5941–5952.
- Neviani, P.; Santhanam, R.; Oaks, J.J.; Eiring, A.M.; Notari, M.; Blaser, B.W.; Liu, S.; Trotta, R.; Muthusamy, N.; Gambacorti-Passerini, C.; et al. FTY720, a new alternative for treating blast crisis chronic myelogenous leukemia and Philadelphia chromosome–positive acute lymphocytic leukemia. J. Clin. Investig. 2007, 117, 2408–2421.
- Lucas, C.M.; Harris, R.J.; Giannoudis, A.; Copland, M.; Slupsky, J.R.; Clark, R.E. Cancerous inhibitor of PP2A (CIP2A) at diagnosis of chronic myeloid leukemia is a critical determinant of disease progression. Blood 2011, 117, 6660–6668.
- Lucas, C.M.; Milani, M.; Butterworth, M.; Carmell, N.; Scott, L.J.; Clark, R.E.; Cohen, G.M.; Varadarajan, S. High CIP2A levels correlate with an antiapoptotic phenotype that can be overcome by targeting BCL-XL in chronic myeloid leukemia. Leukemia 2016, 30, 1273–1281.
- Silvestri, G.; Trotta, R.; Stramucci, L.; Ellis, J.J.; Harb, J.G.; Neviani, P.; Wang, S.; Eisfeld, A.K.; Walker, C.J.; Zhang, B.; et al. Persistence of Drug-Resistant Leukemic Stem Cells and Impaired NK Cell Immunity in CML Patients Depend on MIR300 Antiproliferative and PP2A-Activating Functions. Blood Cancer Discov. 2020, 1, 48–67.
- Neviani, P.; Harb, J.G.; Oaks, J.J.; Santhanam, R.; Walker, C.J.; Ellis, J.J.; Ferenchak, G.; Dorrance, A.M.; Paisie, C.A.; Eiring, A.M.; et al. PP2A-activating drugs selectively eradicate TKI-resistant chronic myeloid leukemic stem cells. J. Clin. Investig. 2013, 123, 4144–4157.
- Wang, S.; Xie, W.; Wang, D.; Peng, Z.; Zheng, Y.; Liu, N.; Dai, W.; Wang, Y.; Wang, Z.; Yang, Y.; et al. Discovery of a small molecule targeting SET-PP2A interaction to overcome BCR-ABLT315I mutation of chronic myeloid leukemia. Oncotarget 2015, 6, 12128–12140.
- Agarwal, A.; MacKenzie, R.J.; Pippa, R.; Eide, C.A.; Oddo, J.; Tyner, J.W.; Sears, R.; Vitek, M.P.; Odero, M.D.; Christensen, D.J.; et al. Antagonism of SET Using OP449 Enhances the Efficacy of Tyrosine Kinase Inhibitors and Overcomes Drug Resistance in Myeloid Leukemia. Clin. Cancer Res. 2014, 20, 2092–2103.
- Laidlaw, K.M.E.; Berhan, S.; Liu, S.; Silvestri, G.; Holyoake, T.L.; Frank, D.A.; Aggarwal, B.; Bonner, M.Y.; Perrotti, D.; Jørgensen, H.G.; et al. Cooperation of imipramine blue and tyrosine kinase blockade demonstrates activity against chronic myeloid leukemia. Oncotarget 2016, 7, 51651–51664.
- Lai, D.; Chen, M.; Su, J.; Liu, X.; Rothe, K.; Hu, K.; Forrest, D.L.; Eaves, C.J.; Morin, G.B.; Jiang, X. PP2A inhibition sensitizes cancer stem cells to ABL tyrosine kinase inhibitors in BCR-ABL+human leukemia. Sci. Transl. Med. 2018, 10, eaan8735.
- Lai, D.; Chen, M.; Su, J.; Liu, X.; Rothe, K.; Hu, K.; Forrest, D.L.; Eaves, C.J.; Morin, G.B.; Jiang, X. Response to Comment on “PP2A inhibition sensitizes cancer stem cells to ABL tyrosine kinase inhibitors in BCR-ABL+ human leukemia”. Sci. Transl. Med. 2019, 11, eaav0819.
- Perrotti, D.; Agarwal, A.; Lucas, C.M.; Narla, G.; Neviani, P.; Odero, M.D.; Ruvolo, P.P.; Verrills, N.M. Comment on “PP2A inhibition sensitizes cancer stem cells to ABL tyrosine kinase inhibitors in BCR-ABL human leukemia”. Sci. Transl. Med. 2019, 11, eaau0416.
- Través, P.G.; Pardo, V.; Pimentel-Santillana, M.; González-Rodríguez, Á.; Mojena, M.; Rico, D.; Montenegro, Y.; Calés, C.; Martín-Sanz, P.; Valverde, A.M.; et al. Pivotal role of protein tyrosine phosphatase 1B (PTP1B) in the macrophage response to pro-inflammatory and anti-inflammatory challenge. Cell Death Dis. 2014, 5, e1125.
- Le Sommer, S.; Morrice, N.; Pesaresi, M.; Thompson, D.; Vickers, M.A.; Murray, G.I.; Mody, N.; Neel, B.G.; Bence, K.K.; Wilson, H.M.; et al. Deficiency in Protein Tyrosine Phosphatase PTP1B Shortens Lifespan and Leads to Development of Acute Leukemia. Cancer Res. 2018, 78, 75–87.
- Alvira, D.; Naughton, R.; Bhatt, L.; Tedesco, S.; Landry, W.D.; Cotter, T.G. Inhibition of Protein-tyrosine Phosphatase 1B (PTP1B) Mediates Ubiquitination and Degradation of Bcr-Abl Protein. J. Biol. Chem. 2011, 286, 32313–32323.
- LaMontagne, K.R.; Hannon, G.; Tonks, N.K. Protein tyrosine phosphatase PTP1B suppresses p210 bcr-abl-induced transformation of Rat-1 fibroblasts and promotes differentiation of K562 cells. Proc. Natl. Acad. Sci. USA 1998, 95, 14094–14099.
- Elgehama, A.; Chen, W.; Pang, J.; Mi, S.; Li, J.; Guo, W.; Wang, X.; Gao, J.; Yu, B.; Shen, Y.; et al. Blockade of the interaction between Bcr-Abl and PTB1B by small molecule SBF-1 to overcome imatinib-resistance of chronic myeloid leukemia cells. Cancer Lett. 2016, 372, 82–88.
- Koyama, N.; Koschmieder, S.; Tyagi, S.; Portero-Robles, I.; Chromic, J.; Myloch, S.; Nürnberger, H.; Rossmanith, T.; Hofmann, W.-K.; Hoelzer, D.; et al. Inhibition of Phosphotyrosine Phosphatase 1B Causes Resistance in BCR-ABL-Positive Leukemia Cells to the ABL Kinase Inhibitor STI571. Clin. Cancer Res. 2006, 12, 2025–2031.
- Gu, C.; Liu, Y.; Yin, Z.; Yang, J.; Huang, G.; Zhu, X.; Li, Y.; Fei, J. Discovery of the Oncogenic Parp1, a Target of bcr-abl and a Potential Therapeutic, in mir-181a/PPFIA1 Signaling Pathway. Mol. Ther. Nucleic Acids 2019, 16, 1–14.
- Chien, W.; Tidow, N.; Williamson, E.A.; Shih, L.-Y.; Krug, U.; Kettenbach, A.; Fermin, A.C.; Roifman, C.M.; Koeffler, H. Characterization of a Myeloid Tyrosine Phosphatase, Lyp, and Its Role in the Bcr-Abl Signal Transduction Pathway. J. Biol. Chem. 2003, 278, 27413–27420.
- Faria, A.V.S.; Clerici, S.P.; de Souza Oliveira, P.F.; Queiroz, K.C.S.; Peppelenbosch, M.P.; Ferreira-Halder, C.V. LMWPTP modulates the antioxidant response and autophagy process in human chronic myeloid leukemia cells. Mol. Cell. Biochem. 2020, 466, 83–89.
- Ferreira, P.A.; Ruela-De-Sousa, R.R.; Queiroz, K.C.S.; Souza, A.C.S.; Milani, R.; Pilli, R.A.; Peppelenbosch, M.P.; Hertog, J.D.; Ferreira, C.V. Knocking Down Low Molecular Weight Protein Tyrosine Phosphatase (LMW-PTP) Reverts Chemoresistance through Inactivation of Src and Bcr-Abl Proteins. PLoS ONE 2012, 7, e44312.
- Naughton, R.; Quiney, C.; Turner, S.D.; Cotter, T.G. Bcr-Abl-mediated redox regulation of the PI3K/AKT pathway. Leukemia 2009, 23, 1432–1440.
- Shimizu, T.; Miyakawa, Y.; Oda, A.; Kizaki, M.; Ikeda, Y. STI571-resistant KT-1 cells are sensitive to interferon-α accompanied by the loss of T-cell protein tyrosine phosphatase and prolonged phosphorylation of Stat1. Exp. Hematol. 2003, 31, 601–608.
- Shimizu, T.; Miyakawa, Y.; Iwata, S.; Kuribara, A.; Tiganis, T.; Morimoto, C.; Ikeda, Y.; Kizaki, M. A novel mechanism for imatinib mesylate (STI571) resistance in CML cell line KT-1: Role of TC-PTP in modulating signals downstream from the BCR-ABL fusion protein. Exp. Hematol. 2004, 32, 1057–1063.
- Mitra, A.; Sasikumar, K.; Parthasaradhi, B.; Radha, V. The tyrosine phosphatase TC48 interacts with and inactivates the oncogenic fusion protein BCR-Abl but not cellular Abl. Biochim. Biophys. Acta Mol. Basis Dis. 2013, 1832, 275–284.
