Escherichia coli filamentous bacteriophages (M13, f1, or fd) have attracted tremendous attention from vaccinologists as a promising immunogenic carrier and vaccine delivery vehicle with vast possible applications in the development of vaccines. The use of fd bacteriophage as an antigen delivery system is based on a modification of bacteriophage display technology. In particular, it is designed to express multiple copies of exogenous peptides (or polypeptides) covalently linked to viral capsid proteins. This study for the first time proposes the use of microparticles (MPs) made of poly (lactic-co-glycolic acid) (PLGA) to encapsulate fd bacteriophage. Using recombinant bacteriophages expressing the ovalbumin (OVA) antigenic determinant, we demonstrated the immunogenicity of the encapsulated bacteriophage after being released by MPs. Our results reveal that encapsulated bacteriophages are stable and retain their immunogenic properties. Bacteriophage-encapsulated PLGA microparticles may thus represent an important tool for the development of different bacteriophage-based vaccine platforms.
- vaccine delivery
- bacteriophage display
- microparticles
- sustained-release
- immunity
- dendritic cells (DC)
Bacteriophages are a powerful platform with outstanding potential in the biomedical and chemical engineering field that have been exploited for diverse applications including theranostics, batteries, drug delivery, and vaccine development [1]. Filamentous bacteriophages are single-strand DNA virions belonging to the Inoviridae family, a sub-group of non-lytic, rod-like shaped Escherichia coli viruses with a repeated and ordered capsid structure, and that includes phages f1, fd, and M13. Fd filamentous bacteriophage is a bio nano-fiber with a modifiable surface that is a promising vehicle for antigen expression. A considerable body of data has been accumulated concerning the molecular basis of structural and functional features of fd bacteriophage. Fd bacteriophage genome is intrinsically rich in deoxycytidylate-phosphate-deoxy guanylate (CpG) regions, which can be recognized by toll-like receptors (TLRs). After activation of TLRs, signaling induces the generation of inflammatory signal mediators such as cytokines, and can develop adaptive immune responses without needing any exogenous adjuvant [2].
The major goal of vaccination is to induce a strong immune response and long-lasting immunity [3]. Due to the lack of an appropriate delivery system, some antigens are unable to induce a strong and effective immune response, and therefore the emergence of an optimal delivery system is of great interest for new vaccine formulations.
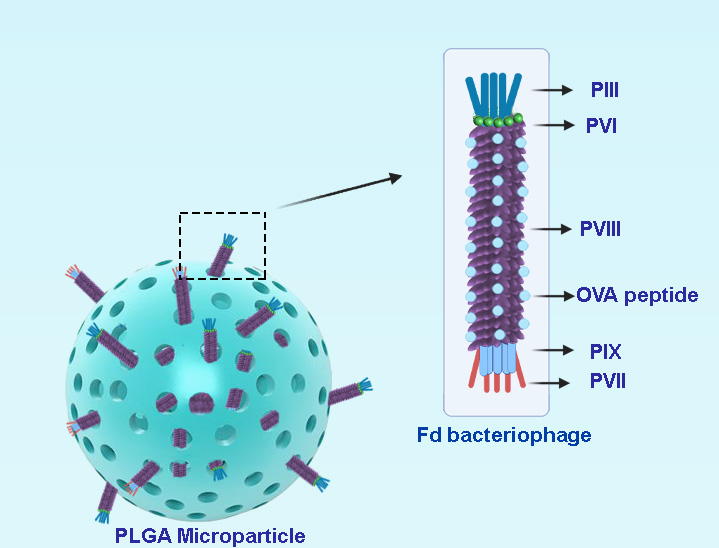
The fd filamentous bacteriophage antigen display system is a vaccine candidate that is able to induce both innate and adaptive immune responses [4]. Based on a modification of phage display technology, fd bacteriophage was engineered to express exogenous peptides in high copy numbers, as fusions to the N-terminus of viral capsid protein pVIII. Recombinant hybrid virions carry multiple copies of pVIII-containing exogenous sequences interspersed with wild-type pVIII copies on the phage coat. Peptide display on filamentous bacteriophages can be employed to present antigenic peptides to antigen presenting cells (APCs) and thus trigger a strong immune response.
The development of genetically engineered bacteriophage preparations with improved pH resistance [5], embedded in hydrogel microspheres [6] or pH-responsive polymers [7] can ameliorate the stability of filamentous bacteriophage vaccines. Additionally, the encapsulation of virions into appropriate polymeric microparticles can improve the half-life of the bacteriophage by protecting it in harsh environments (e.g., the gastrointestinal tract at low pH, if administered orally). Furthermore, the encapsulation in a biodegradable porous system can modulate the release of bacteriophages, leading to a prolonged stimulation of the immune system over time and increasing the immunogenicity of the phage-based vaccine [8]. In this work, PLGA was used as a biodegradable and biocompatible polymer to fabricate microparticles (MPs) through the water in oil in water (W1/O/W2) double-emulsion/solvent evaporation method [9]. However, for several proteins, it has been shown that the presence of a large interface in double emulsion between aqueous and organic solvents such as dichloromethane (DCM) is responsible for protein degradation during emulsification. Furthermore, there are some mechanical stresses applied during fabrication, including shear stress during mixing and agitation. For this reason, we decided to fabricate empty PLGA microparticles then load bacteriophages into their open porosities, in order to avoid antigen degradation and preserve an intact bacteriophage structure.
Figure 1. Schematic presentation of bacteriophage loaded PLGA microparticles.
Bacteriophage-Encapsulated MPs
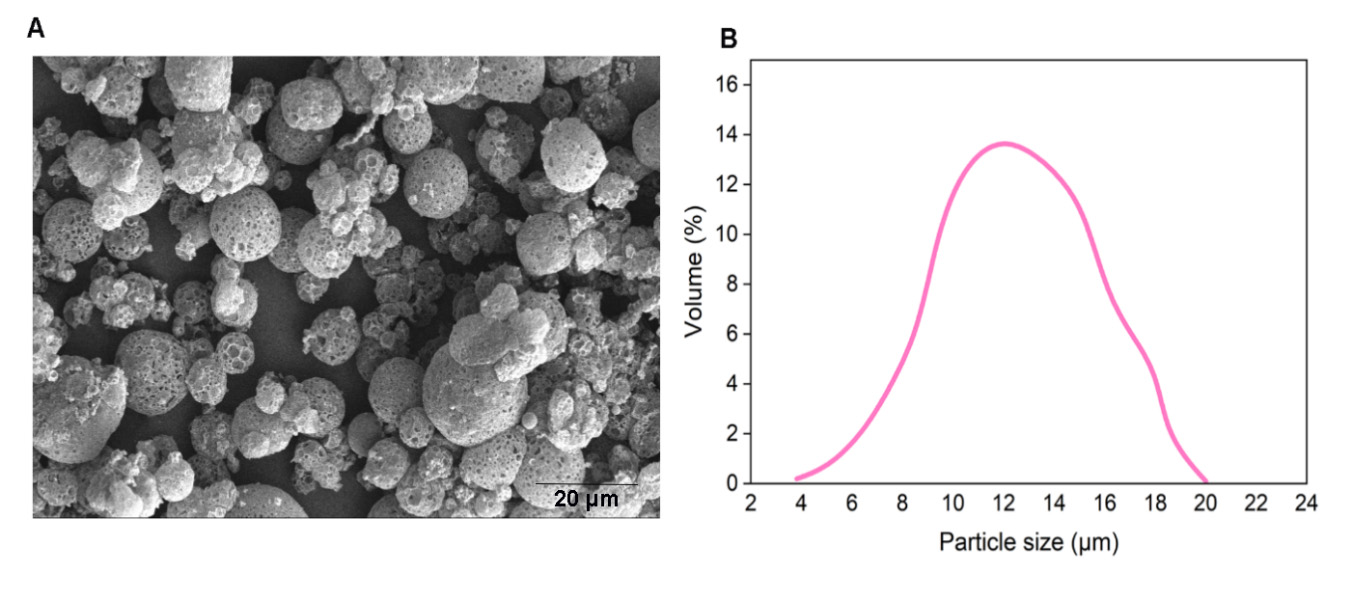
We prepared empty porous MPs of PLGA via water-oil-water double emulsion. Porosities were generated by using ammonium bicarbonate (ABC) in the inner aqueous phase. We generated highly porous empty MPs with an average size of d = 14 µm (Figure 2). In this encapsulation method, filamentous bacteriophages were loaded on the surface of generated empty MPs via incubation at 4 ◦C overnight.
Figure 2. (A) SEM of empty MPs. (B) Size distribution of porous empty MPs.
Antigen-Specific Immune Response to Encapsulated fdOVA Bacteriophage
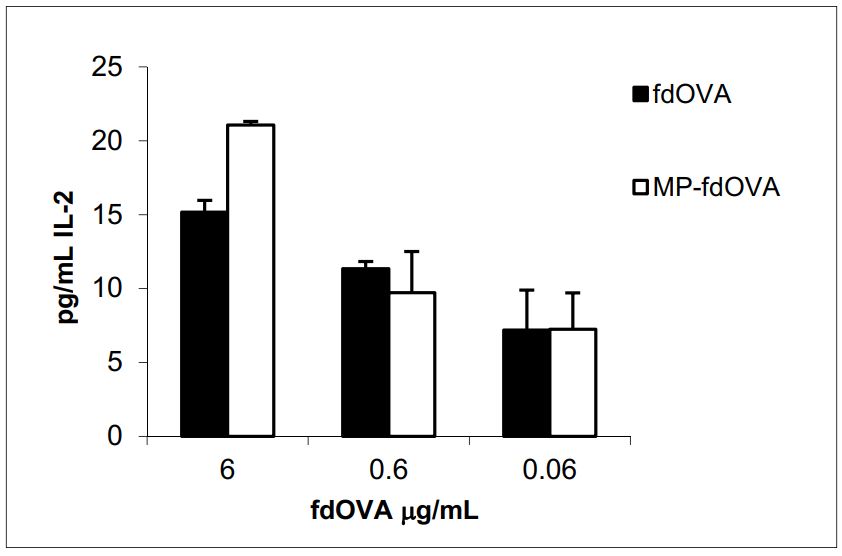
We evaluated the immunological response to the antigen delivered by bacteriophage particles after the encapsulation process. Antigen presentation functions are crucial for the activation of an immune response. Mouse BM-DCs were incubated overnight with free fdOVA bacteriophage or fdOVA bacteriophage prior to release from MPs. DCs were then assessed for their capability to process filamentous bacteriophage and activate OVA (257–264)-specific hybridoma T cell B3Z, a CD8+ T cell line known to produce IL-2 exclusively upon activation of a T cell receptor specific to the OVA (257–264) SIINFEKL peptide in the context of H-2Kb MHC class I complex, expressed on APCs. B3Z activation in response to OVA (257–264) was measured by interleukin-2 (IL-2) released in the culture supernatants. We found that fdOVA bacteriophage released from MPs after post encapsulation was able to be taken and processed by dendritic cells, inducing a B3Z response similar to the one obtained using free fdOVA bacteriophage.
Figure 3. IL-2 release of B3Z hybridoma cell line in response to fdOVA delivering OVA (257–264) SIINFEKL peptide. Bone marrow-derived dendritic cells (BM-DCs) were incubated with graded doses of fdOVA bacteriophage free or released from post-encapsulated MPs. BM-DCs were co-cultured with B3Z hybridoma cells for 40 h and supernatants were assayed in duplicate.
In summary, we present a fabrication method for encapsulating fd filamentous bacteriophage into PLGA polymeric MPs that grants both bacteriophage stability and infectivity, as well as the immunogenicity of the antigen displayed on bacteriophage.
References
- Antonella Prisco; Piergiuseppe De Berardinis; Filamentous Bacteriophage Fd as an Antigen Delivery System in Vaccination. International Journal of Molecular Sciences 2012, 13, 5179-5194, 10.3390/ijms13045179.
- Krystina L. Hess; Christopher M. Jewell; Phage display as a tool for vaccine and immunotherapy development. Bioengineering & Translational Medicine 2019, 5, 10142, 10.1002/btm2.10142.
- Mojgan Allahyari; Elham Mohit; Peptide/protein vaccine delivery system based on PLGA particles. Human Vaccines & Immunotherapeutics 2015, 12, 806-828, 10.1080/21645515.2015.1102804.
- Rossella Sartorius; Luciana D'apice; Maria Trovato; Fausta Cuccaro; Valerio Costa; Maria Giovanna De Leo; Vincenzo Manuel Marzullo; Carmelo Biondo; Sabato D'auria; Maria Antonietta De Matteis; et al.Alfredo CiccodicolaPiergiuseppe De Berardinis Antigen delivery by filamentous bacteriophage fd displaying an anti‐ DEC ‐205 single‐chain variable fragment confers adjuvanticity by triggering a TLR 9‐mediated immune response. EMBO Molecular Medicine 2015, 7, 973-988, 10.15252/emmm.201404525.
- Franklin L. Nobrega; Ana Rita Costa; José F. Santos; Melvin F. Siliakus; Jan W. M. Van Lent; Servé Kengen; Joana Azeredo; Leon D. Kluskens; Genetically manipulated phages with improved pH resistance for oral administration in veterinary medicine. Scientific Reports 2016, 6, 39235, 10.1038/srep39235.
- Yongsheng Ma; Jennifer C. Pacan; Qi Wang; Yongping Xu; Xiaoqing Huang; Anton Korenevsky; P. M. Sabour; Microencapsulation of Bacteriophage Felix O1 into Chitosan-Alginate Microspheres for Oral Delivery. Applied and Environmental Microbiology 2008, 74, 4799-4805, 10.1128/aem.00246-08.
- Gurinder K. Vinner; Goran T. Vladisavljević; Martha R. J. Clokie; Danish J. Malik; Microencapsulation of Clostridium difficile specific bacteriophages using microfluidic glass capillary devices for colon delivery using pH triggered release. PLOS ONE 2017, 12, e0186239, 10.1371/journal.pone.0186239.
- Danish J. Malik; Ilya J Sokolov; Gurinder K. Vinner; Francesco Mancuso; Salvatore Cinquerrui; Goran T. Vladisavljević; Martha R J Clokie; Natalie J Garton; Andrew G F Stapley; Anna Kirpichnikova; et al. Formulation, stabilisation and encapsulation of bacteriophage for phage therapy.. Advances in Colloid and Interface Science 2017, 249, 100-133.
- J. Maxwell Mazzara; Lukasz J. Ochyl; Justin K. Y. Hong; James J. Moon; Mark R. Prausnitz; Steven P. Schwendeman; Self‐healing encapsulation and controlled release of vaccine antigens from PLGA microparticles delivered by microneedle patches. Bioengineering & Translational Medicine 2018, 4, 116-128, 10.1002/btm2.10103.