The emergence of antimicrobial resistance in Gram-negative bacteria poses a huge health challenge. The therapeutic use of polymyxins (i.e., colistin and polymyxin B) is commonplace due to high efficacy and limiting treatment options for multidrug-resistant Gram-negative bacterial infections. Nephrotoxicity and neurotoxicity are the major dose-limiting factors that limit the therapeutic window of polymyxins; nephrotoxicity is a complication in up to ~60% of patients. The emergence of polymyxin-resistant strains or polymyxin heteroresistance is also a limiting factor. These caveats have catalyzed the search for polymyxin combinations that synergistically kill polymyxin-susceptible and resistant organisms and/or minimize the unwanted side effects. Curcumin—an FDA-approved natural product—exerts many pharmacological activities. Recent studies showed that polymyxins–curcumin combinations showed a synergistically inhibitory effect on the growth of bacteria (e.g., Gram-positive and Gram-negative bacteria) in vitro. Moreover, curcumin co-administration ameliorated colistin-induced nephrotoxicity and neurotoxicity by inhibiting oxidative stress, mitochondrial dysfunction, inflammation and apoptosis.
1. Introduction
The World Health Organization
[1] has highlighted antimicrobial resistance as a major global health concern
[1]. As no new classes of antibiotics will be available for Gram-negative ‘superbugs’ in the near future, we have to develop novel polymyxin combination therapies
[2]. Polymyxins were firstly discovered in 1947 from different species of
Bacillus polymyxa [3]. There are five members in the polymyxins family, i.e., polymyxin A, B, C, D and E (also named colistin). Out of these, only polymyxins B and colistin are used in clinical practice, as agents against multi-drug resistant (MDR) Gram-negative pathogens, in particular
Pseudomonas aeruginosa (
P. aeruginosa)
Acinetobacter baumannii (
A. baumannii) and
Klebsiella pneumoniae (
K. pneumoniae). They differs by only one amino acid (a)
[4][5][6][7][8][9][10][11][4,5,6,7,8,9,10,11].
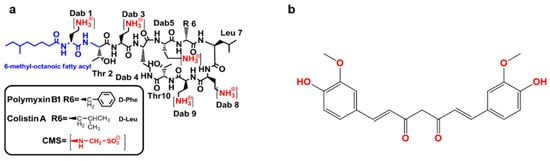
Figure 1. The structure of polymyxin B and colistin (a) and curcumin (b). CMS—colistin methanesulfonate; Thr—threonine; Leu—leucine; Phe—phenylalanine; Dab—α,γ- diaminobutyric acid.
Unlike polymyxin B, which is available in the clinic as the sulfate salt, colistin is used in the clinic as an inactive pro-drug, colistin methanesulfonate (CMS) (a)
[12][13][12,13]. Polymyxin B is available for intravenous, oral and topical use and CMS for parenteral use; both can be delivered by inhalation
[14]. Plasmid carried mobilized colistin resistance (mcr) gene-mediated polymyxin resistance has been increasingly reported in MDR Gram-negatives worldwide. Polymyxin resistance implies a total lack of antibiotics against Gram-negative ‘superbugs’, without the availability of novel antibiotics in the near future, the development of superior polymyxin combinations to combat a ‘post-antibiotic era’ is paramount
[15][16][15,16]. This has brought the effectiveness of polymyxin monotherapy into question. Unfortunately, only increasing the dose of polymyxins to overcome resistance is not an option due to toxic side effects (i.e., nephrotoxicity and neurotoxicity)
[17]. Previous clinical observations showed that the rates of nephrotoxicity occurred in approximately 60% after patients received colistin or polymyxin B therapy
[18]. Based on the narrow therapeutic indices of polymyxins, a key strategy for overcoming resistance and concomitantly ablating unwanted side effects is combining polymyxins with other agents. Although polymyxin-antibiotic combinations exhibit well-confirmed synergistic effects against bacterial growth in vitro
[19][20][19,20]. The clinical findings from randomized, controlled prospective trials have shown that these combinations have no increased benefits compared to their respective monotherapies
[19][20][19,20]. Moreover, some polymyxin-antibiotic combinations may increase the risk of renal failure (e.g., colistin–vancomycin combination)
[20].
Secondary metabolite natural products have been at the forefront of drug candidates for the treatment of cancer, infection and neurodegenerative diseases for decades
[21]. Natural products have been the source of most of our antibacterial armamentarium and efforts over the last 30 years
[22]. Recent studies have shown that combining polymyxins with curcumin has many pharmacological and toxicological benefits over monotherapy with each compound per se including: (1), the in vitro synergy against various strains of MDR bacteria
[23], including polymyxin resistant isolates
[24]; (2), protection against polymyxin-induced nephron- and neuro-toxicity
[25][26][27][25,26,27]; (3), curcumin supplementation improves recovery from the bacterial infection or LPS—induced inflammatory response or sepsis
[28][29][30][31][32][33][28,29,30,31,32,33]. Importantly, human clinical trials indicated that curcumin displays good safety and tolerability
[34][35][36][34,35,36]; oral administration of curcumin at eight grams per day over three months or a single oral dose of 12 grams has no marked adverse effect
[37][38][37,38].
2. Synergistic Antibacterial Effects of the Polymyxin in Combination with Curcumin
The putative primary mechanism of action of polymyxins is the disruption of the Gram-negative outer membrane through an initial electrostatic interaction that results in cation displacement (Ca
2+ and Mg
2+) and subsequent binding to the lipid A component of LPS, leading to leakage of the cytoplasmic content and ultimately causing cell death
[39][72]. As a secondary killing effect, polymyxins induce ROS
[40][73]. The inhibition of bacterial respiration has also been associated with the bacterial killing action of polymyxins
[41][74].
Polymyxin resistance is conferred by mcr-mediated structural modifications of LPS that act to reduce the negative charge of the outer membrane, which in turn repels the polymyxin molecule
[42][75]. The two main LPS modifications conferring polymyxin resistance are the addition of phosphoethanolamine (PetN) and 4-amino-4-deoxy-L-arabinose (AraN) to the lipid A
[43][76]. Recently, plasmid-mediated colistin resistance was reported in
Enterobacteriaceae due to the PetN transferase mcr-1
[15].
In vitro time-kill curves for polymyxin B in combination with curcumin showed a marked synergetic effect against antibiotic-susceptible and—resistant Gram-positive (
Enterococci,
S. aureus and streptococci) and Gram-negative (
A. baumannii,
E. coli,
P. aeruginosa and
S. maltophilia) bacterial isolates associated isolated from traumatic wound infections
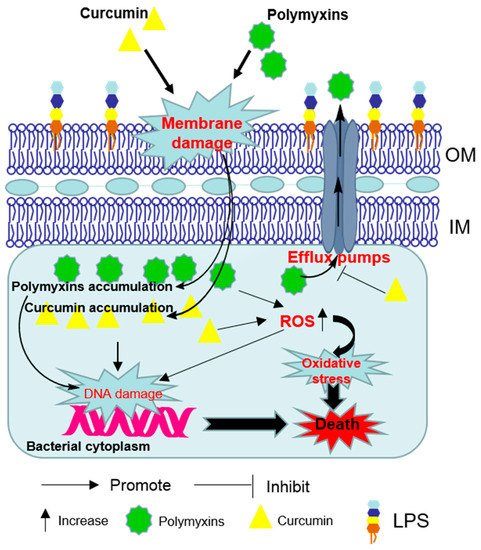
[23]. The synergistic effect may result from the ability of polymyxin to permeabilize the outer membrane which facilitates the access of greater concentrations of curcumin to its intracellular targets ()
[24]. Curcumin may also overcome the polymyxin-resistance by inhibiting the activities of efflux pumps where located in outer membrane (OM) or inner membranes (IM)
[24].
Figure 2. Schematic diagram depicting the synergistic bacterial killing mechanism of the polymyxins–curcumin combination. Polymyxins permeabilize the Gram-negative outer membrane and thereby promote curcumin enter the bacterial cell. Bacterial cell death finally ensues via reactive oxygen species (ROS) production, oxidative stress and DNA damage. Curcumin may also overcome the polymyxin-resistance by disturbing the activities of efflux pumps. OM—outer membrane; IM—inner membranes; LPS—lipopolysaccharide.
3. Polymyxin-Induced Nephrotoxicity and Protective Effect of Curcumin
Nephrotoxicity is the major dose-limiting factor of polymyxins in clinical practice and it occurs in up to 60% of patients after intravenous administration
[18]. Clinical manifestations include a decrease in creatinine (CRE) clearance, as well as proteinuria, oliguria (low output of urine) and cylindruria (presence of casts in the urine)
[18][44][45][18,77,78]. Pathologic characteristics of colistin or polymyxin B-induced nephrotoxicity were included tubular dilation and tubular epithelial cell vacuolation and necrosis, tubular casts and inflammatory cell infiltration
[46][47][48][79,80,81]. In clinical practice, serum CRE and blood urea nitrogen (BUN) are usually employed as the biomarkers of renal function. However, their use as the biomarkers has some limitations in polymyxin therapy, such as dependence on nutrition, age, sex and body mass and is likely to reflect already advanced damage. Studies in animal models showed that kidney injury molecule 1 (KIM-1), Cystatin C and α-glutathione
S-transferase may be more reliable markers than CRE or BUN to monitor renal function during polymyxin therapy
[49][50][82,83].
Our understanding of the molecular mechanisms underlying polymyxin-induced nephrotoxicity have significantly advanced in the past 15 years owing to the pioneering work of the Li group from Monash University, Australia
[51][52][53][54][55][56][57][58][59][60][61][62][63][84,85,86,87,88,89,90,91,92,93,94,95,96]. Their recent studies have revealed significant renal accumulation of polymyxins using immunostaining, fluorescence microscopy, mass spectrometry imaging and X-ray fluorescence microscopy (XFM)
[51][52][53][54][84,85,86,87]. Predominant accumulation of polymyxin B was distinct in the renal cortex, in particular the renal proximal tubular cells, but much less in the distal tubular cells
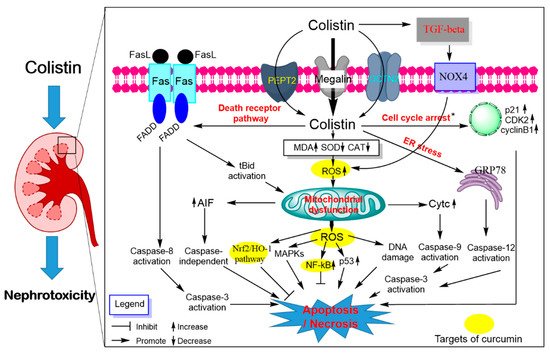
[51][52][84,85]. Moreover, polymyxins substantially accumulate in proximal tubular cells via receptor-mediated endocytosis mainly by megalin
[64][65][97,98], human peptide transporter 2 (PEPT2,
syn. SLC15A2)
[58][63][91,96] and the carnitine/organic cation transporter 2 (OCTN2,
syn. SLC22A5)
[66][99] (). Inhibitors of these receptors were shown to block the uptake of polymyxins and then attenuate colistin or polymyxin B- induced nephrotoxicity in mice
[58][64][67][91,97,100].
Figure 3. Pathways involved in polymyxin-induced nephrotoxicity and the potential nephro-protective mechanism of curcumin. Colistin extensively accumulates in kidney tubular cells via receptor-mediated uptake, megalin, peptide transporter 2 (PEPT2) and the carnitine/organic cation transporter 2 (OCTN2). Intracellular colistin accumulation in kidney tubular cells induces the production of ROS, mitochondrial dysfunction and triggers a series of signaling cascades (e.g., cell cycle arrest, p53, nuclear factor kappa B [NF-κB] and mitogen-activated protein kinase [MAPK], nuclear factor erythroid 2-related factor 2 [Nrf2]/heme oxygenase-1 [HO-1] pathways). The activation of the transforming growth factor (TGF)-β/nicotinamide adenine dinucleotide 3-phosphate oxidase-4 (NOX-4) pathway contributes to oxidative stress by promoting ROS production. All three major apoptosis pathways (e.g., mitochondrial, death receptor and endoplasmic reticulum pathways) participated in colistin-induced nephrotoxicity. Curcumin treatment improved colistin-induced nephrotoxicity by targeting the NF-kB mediated inflammatory response, oxidative stress and upregulating the antioxidant Nrf2/HO-1 pathways.
Polymyxin treatment can lead to DNA damage and apoptotic cell death in renal tubular cells in the cultured cell model or in vivo animal model
[60][68][69][70][93,101,102,103] (). Our group has been pioneering this area in reporting a series of studies showing that polymyxin B or colistin-induced apoptosis involved the death receptor (upregulation of Fas, FasL and Fas-associated death domain [FADD]), mitochondrial (downregulation of B-cell lymphoma 2 [Bcl-2] and upregulation of cytochrome C [CytC] and bcl-2-like protein 4 [Bax] and endoplasmic reticulum (upregulation of activating transcription factor 6 [ATF6], glucose-regulated protein 78 [GRP78], caspase-12 and growth arrest and DNA damage-inducible gene 153 [GADD153/CHOP]) pathways in cultured renal tubular cells
[68][101] and the kidney tissue of mouse
[60][93]. Polymyxin B or colistin treatment can induce loss of mitochondrial membrane potential, morphology changes and the generation of ROS mediated oxidative damage in a concentration-dependent manner
[60][68][93,101]. Significant Elevated protein expression levels of the p53, cyclin-dependent kinase 2 (CDK2) and phosphorylated Jun N-terminal kinase (JNK) and p38 mitogen-activated protein kinase (MAPK) and autophagy were detected in kidney tissues of the colistin-treated mice
[60][93]. The lipid peroxidation marker malondialdehyde (MDA), nitrative stress-related nitric oxide (NO) and inducible nitric oxide synthase activities and inflammation response were significantly increased in the kidney tissues from colistin-treated mice
[47][71][80,104]. Additionally, the activation of transforming growth factor (TGF)-β/nicotinamide adenine dinucleotide 3-phosphate oxidase-4 (NOX-4) pathway contributes to the production of ROS in kidneys of mice; inhibition of TGF-β expression markedly attenuated colistin-induced renal damage in mice, indicating that TGF-β/NOX-4 pathway may play a critical role in colistin-induced nephrotoxicity
[72][105].
Curcumin has been shown to protect against colistin-induced nephrotoxicity via inhibition of oxidative stress, inflammation and apoptosis. A previous study reported that curcumin supplementation (e.g., at 200 mg/kg/day for 3 days) markedly protects against glycerol-induced acute kidney injury by inhibiting oxidative damage and activating Nrf2 pathway in rats
[73][106]. A similar molecular mechanism has also been detected in the protection of curcumin against gentamicin-induced nephrotoxicity in rats
[74][107]. Consistent with these findings, curcumin supplementation (e.g., at 200 mg/kg/day for 6 days) can also markedly improve colistin-induced nephrotoxicity via the inhibition of oxidative stress, apoptosis, NO signaling and inflammatory response (i.e., decreases the tumor necrosis factor-α [TNF-α], interleukin-6 [IL-6] in the kidney tissue) in a rat model
[27]. Our recent data reveal that curcumin after oral administration at 50 and 200 mg/kg/day for 7 days can be detected and accumulated in multiple organs of mice, including kidney tissues
[25]. It was shown that curcumin can promote Nrf2 translocation into nuclei through modification of cysteine sulfhydryl groups in Kelch-like ECH-associated protein 1 (Keap1), a principal negative regulator of Nrf2 in the cytoplasm. The binding of Nrf2 and antioxidant responsive element (ARE) in nuclei activated the transcription activity of ARE that mediates the expression of antioxidant gene such as heme oxygenase-1 (HO-1), CAT and SOD
[75][108]. Indeed, curcumin can directly activate Nrf2/ARE pathway and protects against oxidative stress-mediated apoptotic cell death
[76][109]. Therefore, the activation of Nrf2/HO-1 may contribute to the protective effect of curcumin against colistin–oxidativedamage in the kidney tissues. Besides, it has been reported that curcumin supplementation can protect some drugs (e.g., cisplatin, glycerol and doxorubicin) or toxins (e.g., potassium dichromate, maleate and arsenic)-induced nephrotoxicity by inhibiting oxidative stress, ER stress, inflammatory response, NO pathway, ferroptosis, p53 pathway, MAPK, AMP-activated protein kinase (AMPK) pathways, Akt pathway or activation of autophagy
[73][77][78][79][80][81][82][83][106,110,111,112,113,114,115,116]. The previous study showed that the antioxidant ascorbic acid can protect against colistin-induced nephrotoxicity and cellular apoptosis. Meanwhile, ascorbic acid altered the pharmacokinetics of colistin in a rat model, the total body clearance of colistin decreased from 3.78 ± 0.36 mL/min/kg (colistin alone group) to 2.46 ± 0.57 mL/min/kg (ascorbic acid + colistin co-treatment group), and the half-life of plasma colistin concentration increased from 1.20 ± 0.23 (colistin alone group) h to 3.91 ± 0.42 h (ascorbic acid + colistin co-treatment group)
[61][94].