You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 2 by Catherine Yang and Version 1 by Ramon Garcia-Sanz.
Immunosuppression is a common feature of multiple myeloma (MM) patients and has been associated with disease evolution from its precursor stages. MM cells promote immunosuppressive effects due to both the secretion of soluble factors, which inhibit the function of immune effector cells, and the recruitment of immunosuppressive populations. Alterations in the expression of surface molecules are also responsible for immunosuppression.
- multiple myeloma
- immune system
- immunosuppression
1. Introduction
Monoclonal antibodies (mAbs) have emerged as a backbone therapy for many B-cell tumors, due to their high efficacy and good tolerability. However, the development of effective mAbs for the treatment of MM has been tough, since the discovery of target molecules unique for all MM cells resulted challenging. Up to date, there are three naked mAbs and one antibody–drug conjugate (ADC) approved for the treatment of MM. Their main mechanisms of action can be found in Figure 21.
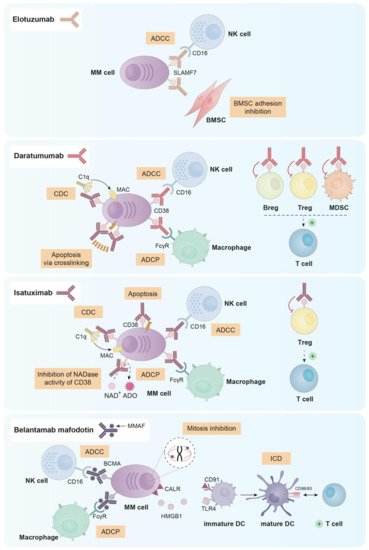
Figure 21. Principal mechanisms of action of the three naked mAbs (elotuzumab, daratumumab and isatuximab) and the ADC (belantamab mafodotin) approved for the treatment of MM.
2. Elotuzumab
Elotuzumab (anti-SLAMF7) was the first mAb approved by the US Food and Drug Administration (FDA) for the treatment of MM. In particular, elotuzumab was first approved in combination with lenalidomide and dexamethasone for relapsed/refractory myeloma patients who had received one to three prior therapies [117][1]. SLAMF7, also known as CS1, is a cell surface molecule expressed in plasma cells, CD8+ cytotoxic T lymphocytes, activated B cells, NK cells and mature DCs [118,119,120,121][2][3][4][5]. In the context of MM, SLAMF7 is expressed in both primary malignant plasma cells and in almost all MM cell lines. In addition, soluble SLAMF7 has been detected in serum of MM patients presenting a direct correlation with disease stage [118,121][2][5].
Elotuzumab is a humanized IgG1 mAb that inhibits MM cell adhesion to BMSCs, which may reverse the protective effect provided by the bone marrow microenvironment to myeloma cells. Additionally, elotuzumab is able to induce antibody dependent cellular cytotoxicity (ADCC) mediated by NK cells in both MM cell lines and primary plasma cells from myeloma patients (either newly diagnosed or resistant to conventional therapies) [121][5].
Since NK cells and a small subset of activated lymphocytes express SLAMF7, elotuzumab is able to activate ex vivo different subsets of peripheral blood mononuclear cells (PBMCs) from myeloma patients and healthy donors. Indeed, elotuzumab selectively activated the subpopulation of CD56dim NK cells, upregulating CD69, CD11b and CD54 and downregulating CD16 expression and resulting in the killing of myeloma cells via a CD16-independent mechanism [122][6]. Moreover, elotuzumab also activated monocytes as evidenced by the up-regulation of SLAMF7, HLA-DR and CD54 [123][7].
3. Daratumumab
Daratumumab is an anti-CD38 mAb that was approved in 2015 by the FDA for MM patients who had received at least three prior lines of therapy or for patients double refractory to proteasome inhibitors and immunomodulatory agents [124,125][8][9]. Besides, it has been recently approved for NDMM patients ineligible for stem-cell transplantation [126][10]. CD38 is expressed in different cell subsets from hematopoietic and non-hematopoietic lineages. Regarding the first, CD38 is expressed in Tregs, circulating monocytes, CD4+ and CD8+ T cells, NK cells, granulocytes/neutrophils, B cell precursors and in terminally differentiated plasma cells from healthy donors [127,128,129][11][12][13]. In the context of MM, CD138+ malignant plasma cells express higher levels of CD38 than other immune subsets and normal plasma cells [130][14]. Moreover, CD38 is also expressed by osteoclasts in the tumor niche [131][15].
Daratumumab was first selected from a panel of 42 human anti-CD38 mAbs for being effective in killing MM cells via complement dependent cytotoxicity (CDC) and ADCC [132][16]. Further studies in vitro, ex vivo and in vivo demonstrated that daratumumab was also able to induce programmed cell death in the presence of crosslinking agents (both F(ab)2 fragments and Fcγ receptor-expressing cells) [133][17], and antibody dependent cellular phagocytosis (ADCP) [134][18].
Given that different immune cell subsets express CD38, daratumumab treatment has an impact on them. In fact, it has been described that MM patients treated with daratumumab both in monotherapy and in combination with lenalidomide and dexamethasone, present a decrease in absolute cell count in NK cells (from 10% to 2%), MDSCs, Bregs and Tregs. On the contrary, other immune populations, such as CD4+ and CD8+ T cells showed increased numbers [135,136,137,138][19][20][21][22]. Despite the decrease in NK cell number observed after daratumumab treatment, according to Casneuf et al. the remaining NK cells seemed to be able to contribute to the clinical efficacy of the drug [137][21]. Furthermore, daratumumab has been reported to induce NK cell activation and degranulation as observed by the upregulation of CD69, CD107a and IFN-γ in this cell subset [139][23].
4. Isatuximab
Isatuximab is a humanized IgG1 anti-CD38 mAb that has been recently approved (March 2020) in combination with pomalidomide and dexamethasone for MM patients who had previously received at least two lines of therapy [140][24]. Isatuximab exerts its antimyeloma effect through different mechanisms. First, and unlike daratumumab, isatuximab has shown proapoptotic activity against myeloma cells expressing high levels of CD38 without any cross-linking agents [141,142][25][26]. Moreover, isatuximab also presents immune-mediated cytotoxic effects, such as, the induction of strong CDC, potent ADCC and ADCP [141,143][25][27]. In contrast to daratumumab, isatuximab completely inhibits the NADase activity of CD38, which may mitigate the immunosuppressive microenvironment in the bone marrow of MM patients [144,145,146][28][29][30].
As observed with daratumumab, isatuximab is able to suppress Tregs. In fact, in PBMCs from both healthy donors and MM patients treated with isatuximab in vitro, the percentage of Tregs was reduced while the percentage of effector T cells increased. This reduction of Treg frequency was more significant in cells from MM patients than from healthy donors, probably due to the higher expression of CD38 observed in patients’ Tregs [127][11]. Furthermore, isatuximab upregulated the activation molecules CD107a and IFNγ in monocytes, CD8+ T cells and NK cells not only from healthy donors but also from myeloma patients, augmenting the cytotoxic functions of these three cell subsets both in the presence and in the absence of CD38+ target cells [127,129][11][13]. Additionally, Moreno et al. observed that isatuximab depleted in vitro CD38high B-lymphocyte precursors, basophils and NK cells [143][27]. In fact, the NK cell depletion observed after isatuximab treatment seems to be generated through activation followed by exhaustion of these cells [143][27].
5. Belantamab Mafodotin
Belantamab mafodotin (GSK2857916) is an afucosylated, humanized IgG1 anti-B-cell maturation antigen (BCMA) mAb conjugated with monomethyl auristatin F (MMAF), which is a tubulin polymerization inhibitor [147][31]. Both parts (anti-BCMA antibody and MMAF toxin) are linked through a non-cleavable maleimidocaproyl linker, which provides better plasma stability of the compound without losing any property and without any nonspecific toxicity [148][32]. Belantamab mafodotin is the first anti-BCMA ADC approved by the FDA as a single agent for relapsed/refractory multiple myeloma (RRMM) patients who have received at least four prior therapies [149][33]. BCMA, also known as TNFRSF-17, is selectively induced during plasma cell differentiation being almost absent on naïve and memory B cells [150,151][34][35]. BCMA is expressed by several myeloma cell lines [152][36] and BCMA mRNA is commonly expressed at high levels in primary malignant plasma cells [153][37].
Belantamab mafodotin exerts its antimyeloma effect through four known mechanisms: (i) ADCC mediated by NK cells; (ii) recruitment of macrophages to promote ADCP; (iii) disruption of microtubules and subsequent G2/M cell-cycle arrest followed by apoptosis after the release of the MMAF toxin in the cytoplasm of myeloma cells [147][31] and (iv) induction of immunogenic cell death (ICD) [148][32], which is a mechanism characterized by the ability of dying cells to elicit robust adaptive immune responses against altered self-antigens or cancer-derived neo-epitopes [154][38]. In relation to the latter mechanism, preliminary data indicates that treatment of myeloma cells with belantamab mafodotin promotes the exposure of calreticulin (CALR) on their surface and the release of HMGB1, which subsequently induce the maturation and activation of DCs and eventually the activation of T cells [148][32].
References
- : FDA-Approved Drugs. Available online: (accessed on 9 November 2020).
- Hsi, E.D.; Steinle, R.; Balasa, B.; Szmania, S.; Draksharapu, A.; Shum, B.P.; Huseni, M.; Powers, D.; Nanisetti, A.; Zhang, Y.; et al. CS1, a Potential New Therapeutic Antibody Target for the Treatment of Multiple Myeloma. Clin. Cancer Res. 2008, 14, 2775–2784.
- Boles, K.S.; Mathew, P.A. Molecular Cloning of CS1, a Novel Human Natural Killer Cell Receptor Belonging to the CD2 Subset of the Immunoglobulin Superfamily. Immunogenetics 2001, 52, 302–307.
- Bouchon, A.; Cella, M.; Grierson, H.L.; Cohen, J.I.; Colonna, M. Cutting Edge: Activation of NK Cell-Mediated Cytotoxicity by a SAP-Independent Receptor of the CD2 Family. J. Immunol. 2001, 167, 5517–5521.
- Tai, Y.-T.; Dillon, M.; Song, W.; Leiba, M.; Li, X.-F.; Burger, P.; Lee, A.I.; Podar, K.; Hideshima, T.; Rice, A.G.; et al. Anti-CS1 Humanized Monoclonal Antibody HuLuc63 Inhibits Myeloma Cell Adhesion and Induces Antibody-Dependent Cellular Cytotoxicity in the Bone Marrow Milieu. Blood 2008, 112, 1329–1337.
- Collins, S.M.; Bakan, C.E.; Swartzel, G.D.; Hofmeister, C.C.; Efebera, Y.A.; Kwon, H.; Starling, G.C.; Ciarlariello, D.; Bhaskar, S.; Briercheck, E.L.; et al. Elotuzumab Directly Enhances NK Cell Cytotoxicity against Myeloma via CS1 Ligation: Evidence for Augmented NK Cell Function Complementing ADCC. Cancer Immunol. Immunother. 2013, 62, 1841–1849.
- Balasa, B.; Huseni, M.; Cherukuri, J.; Steinle, R.; Nanisetti, A.; Afar, D.; Hsi, E.; Vexler, V. Elotuzumab (HuLuc63) Activates CD56dim Natural Killer Cells and Monocytes Resulting in the Release of IP-10 and MCP-1. Blood 2008, 112, 108.
- Lokhorst, H.M.; Plesner, T.; Laubach, J.P.; Nahi, H.; Gimsing, P.; Hansson, M.; Minnema, M.C.; Lassen, U.; Krejcik, J.; Palumbo, A.; et al. Targeting CD38 with Daratumumab Monotherapy in Multiple Myeloma. N. Engl. J. Med. 2015, 373, 1207–1219.
- Lonial, S.; Weiss, B.M.; Usmani, S.Z.; Singhal, S.; Chari, A.; Bahlis, N.J.; Belch, A.; Krishnan, A.; Vescio, R.A.; Mateos, M.V.; et al. Daratumumab Monotherapy in Patients with Treatment-Refractory Multiple Myeloma (SIRIUS): An Open-Label, Randomised, Phase 2 Trial. Lancet 2016, 387, 1551–1560.
- Mateos, M.-V.; Dimopoulos, M.A.; Cavo, M.; Suzuki, K.; Jakubowiak, A.; Knop, S.; Doyen, C.; Lucio, P.; Nagy, Z.; Kaplan, P.; et al. Daratumumab plus Bortezomib, Melphalan, and Prednisone for Untreated Myeloma. N. Engl. J. Med 2018, 378, 518–528.
- Feng, X.; Zhang, L.; Acharya, C.; An, G.; Wen, K.; Qiu, L.; Munshi, N.C.; Tai, Y.-T.; Anderson, K.C. Targeting CD38 Suppresses Induction and Function of T Regulatory Cells to Mitigate Immunosuppression in Multiple Myeloma. Clin. Cancer Res. 2017, 23, 4290–4300.
- Chillemi, A. CD38 and Bone Marrow Microenvironment. Front. Biosci. 2014, 19, 152.
- Zhu, C.; Song, Z.; Wang, A.; Srinivasan, S.; Yang, G.; Greco, R.; Theilhaber, J.; Shehu, E.; Wu, L.; Yang, Z.-Y.; et al. Isatuximab Acts through Fc-Dependent, Independent, and Direct Pathways to Kill Multiple Myeloma Cells. Front. Immunol. 2020, 11, 1771.
- Ghose, J.; Viola, D.; Terrazas, C.; Caserta, E.; Troadec, E.; Khalife, J.; Gunes, E.G.; Sanchez, J.; McDonald, T.; Marcucci, G.; et al. Daratumumab Induces CD38 Internalization and Impairs Myeloma Cell Adhesion. Oncoimmunology 2018, 7, e1486948.
- Iqbal, J.; Zaidi, M. Extracellular NAD+ Metabolism Modulates Osteoclastogenesis. Biochem. Biophys. Res. Commun. 2006, 349, 533–539.
- de Weers, M.; Tai, Y.-T.; van der Veer, M.S.; Bakker, J.M.; Vink, T.; Jacobs, D.C.H.; Oomen, L.A.; Peipp, M.; Valerius, T.; Slootstra, J.W.; et al. Daratumumab, a Novel Therapeutic Human CD38 Monoclonal Antibody, Induces Killing of Multiple Myeloma and Other Hematological Tumors. J. Immunol. 2011, 186, 1840–1848.
- Overdijk, M.B.; Jansen, J.H.M.; Nederend, M.; Lammerts van Bueren, J.J.; Groen, R.W.J.; Parren, P.W.H.I.; Leusen, J.H.W.; Boross, P. The Therapeutic CD38 Monoclonal Antibody Daratumumab Induces Programmed Cell Death via Fcγ Receptor-Mediated Cross-Linking. J. Immunol. 2016, 197, 807–813.
- Overdijk, M.B.; Verploegen, S.; Bögels, M.; van Egmond, M.; Lammerts van Bueren, J.J.; Mutis, T.; Groen, R.W.J.; Breij, E.; Martens, A.C.M.; Bleeker, W.K.; et al. Antibody-Mediated Phagocytosis Contributes to the Anti-Tumor Activity of the Therapeutic Antibody Daratumumab in Lymphoma and Multiple Myeloma. MAbs 2015, 7, 311–321.
- Krejcik, J.; Frerichs, K.A.; Nijhof, I.S.; van Kessel, B.; van Velzen, J.F.; Bloem, A.C.; Broekmans, M.E.C.; Zweegman, S.; van Meerloo, J.; Musters, R.J.P.; et al. Monocytes and Granulocytes Reduce CD38 Expression Levels on Myeloma Cells in Patients Treated with Daratumumab. Clin. Cancer Res. 2017, 23, 7498–7511.
- Wang, Y.; Zhang, Y.; Hughes, T.; Zhang, J.; Caligiuri, M.A.; Benson, D.M.; Yu, J. Fratricide of NK Cells in Daratumumab Therapy for Multiple Myeloma Overcome by Ex Vivo Expanded Autologous NK Cells. Clin. Cancer Res. 2018, 24, 4006–4017.
- Casneuf, T.; Xu, X.S.; Adams, H.C.; Axel, A.E.; Chiu, C.; Khan, I.; Ahmadi, T.; Yan, X.; Lonial, S.; Plesner, T.; et al. Effects of Daratumumab on Natural Killer Cells and Impact on Clinical Outcomes in Relapsed or Refractory Multiple Myeloma. Blood Adv. 2017, 1, 2105–2114.
- Krejcik, J.; Casneuf, T.; Nijhof, I.S.; Verbist, B.; Bald, J.; Plesner, T.; Syed, K.; Liu, K.; van de Donk, N.W.C.J.; Weiss, B.M.; et al. Daratumumab Depletes CD38+ Immune Regulatory Cells, Promotes T-Cell Expansion, and Skews T-Cell Repertoire in Multiple Myeloma. Blood 2016, 128, 384–394.
- Viola, D.; Dona, A.; Caserta, E.; Troadec, E.; Besi, F.; McDonald, T.; Ghoda, L.; Gunes, E.G.; Sanchez, J.F.; Khalife, J.; et al. Daratumumab Induces Mechanisms of Immune Activation through CD38+ NK Cell Targeting. Leukemia 2020.
- Dhillon, S. Isatuximab: First Approval. Drugs 2020, 80, 905–912.
- Deckert, J.; Wetzel, M.-C.; Bartle, L.M.; Skaletskaya, A.; Goldmacher, V.S.; Vallée, F.; Zhou-Liu, Q.; Ferrari, P.; Pouzieux, S.; Lahoute, C.; et al. SAR650984, a Novel Humanized CD38-Targeting Antibody, Demonstrates Potent Antitumor Activity in Models of Multiple Myeloma and Other CD38+ Hematologic Malignancies. Clin. Cancer Res. 2014, 20, 4574–4583.
- Jiang, H.; Acharya, C.; An, G.; Zhong, M.; Feng, X.; Wang, L.; Dasilva, N.; Song, Z.; Yang, G.; Adrian, F.; et al. SAR650984 Directly Induces Multiple Myeloma Cell Death via Lysosomal-Associated and Apoptotic Pathways, Which Is Further Enhanced by Pomalidomide. Leukemia 2016, 30, 399–408.
- Moreno, L.; Perez, C.; Zabaleta, A.; Manrique, I.; Alignani, D.; Ajona, D.; Blanco, L.; Lasa, M.; Maiso, P.; Rodriguez, I.; et al. The Mechanism of Action of the Anti-CD38 Monoclonal Antibody Isatuximab in Multiple Myeloma. Clin. Cancer Res. 2019, 25, 3176–3187.
- Kennedy, B.E.; Sadek, M.; Elnenaei, M.O.; Reiman, A.; Gujar, S.A. Targeting NAD+ Synthesis to Potentiate CD38-Based Immunotherapy of Multiple Myeloma. Trends Cancer 2020, 6, 9–12.
- Martin, T.G.; Corzo, K.; Chiron, M.; van de Velde, H.; Abbadessa, G.; Campana, F.; Solanki, M.; Meng, R.; Lee, H.; Wiederschain, D.; et al. Therapeutic Opportunities with Pharmacological Inhibition of CD38 with Isatuximab. Cells 2019, 8, 1522.
- Lammerts van Bueren, J.; Jakobs, D.; Kaldenhoven, N.; Roza, M.; Hiddingh, S.; Meesters, J.; Voorhorst, M.; Gresnigt, E.; Wiegman, L.; Ortiz Buijsse, A.; et al. Direct in Vitro Comparison of Daratumumab with Surrogate Analogs of CD38 Antibodies MOR03087, SAR650984 and Ab79. Blood 2014, 124, 3474.
- Tai, Y.-T.; Mayes, P.A.; Acharya, C.; Zhong, M.Y.; Cea, M.; Cagnetta, A.; Craigen, J.; Yates, J.; Gliddon, L.; Fieles, W.; et al. Novel Anti–B-Cell Maturation Antigen Antibody-Drug Conjugate (GSK2857916) Selectively Induces Killing of Multiple Myeloma. Blood 2014, 123, 3128–3138.
- Montes de Oca, R.; Bhattacharya, S.; Vitali, N.; Patel, K.; Kaczynski, H.; Shi, H.Z.; Blackwell, C.; Seestaller-Wehr, L.; Cooper, D.; Jackson, H.; et al. The Anti-BCMA Antibody-Drug Conjugate GSK2857916 Drives Immunogenic Cell Death and Immune-Mediated Anti-Tumor Responses, and in Combination with an OX40 Agonist Potentiates in Vivo Activity. EHA Libr. 2019, 3, 231.
- FDA Granted Accelerated Approval to Belantamab Mafodotin-Blmf for Multiple Myeloma. Available online: (accessed on 11 January 2021).
- Avery, D.T.; Kalled, S.L.; Ellyard, J.I.; Ambrose, C.; Bixler, S.A.; Thien, M.; Brink, R.; Mackay, F.; Hodgkin, P.D.; Tangye, S.G. BAFF Selectively Enhances the Survival of Plasmablasts Generated from Human Memory B Cells. J. Clin. Investig. 2003, 112, 286–297.
- Chiu, A.; Xu, W.; He, B.; Dillon, S.R.; Gross, J.A.; Sievers, E.; Qiao, X.; Santini, P.; Hyjek, E.; Lee, J.; et al. Hodgkin Lymphoma Cells Express TACI and BCMA Receptors and Generate Survival and Proliferation Signals in Response to BAFF and APRIL. Blood 2007, 109, 729–739.
- Novak, A.J.; Darce, J.R.; Arendt, B.K.; Harder, B.; Henderson, K.; Kindsvogel, W.; Gross, J.A.; Greipp, P.R.; Jelinek, D.F. Expression of BCMA, TACI, and BAFF-R in Multiple Myeloma: A Mechanism for Growth and Survival. Blood 2004, 103, 689–694.
- Claudio, J.O.; Masih-Khan, E.; Tang, H.; Gonçalves, J.; Voralia, M.; Li, Z.H.; Nadeem, V.; Cukerman, E.; Francisco-Pabalan, O.; Liew, C.C.; et al. A Molecular Compendium of Genes Expressed in Multiple Myeloma. Blood 2002, 100, 2175–2186.
- Galluzzi, L.; Buqué, A.; Kepp, O.; Zitvogel, L.; Kroemer, G. Immunogenic Cell Death in Cancer and Infectious Disease. Nat. Rev. Immunol. 2017, 17, 97–111.
More
