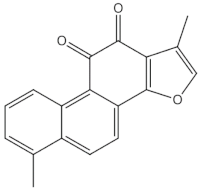
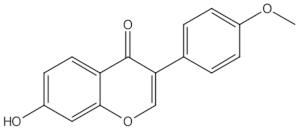
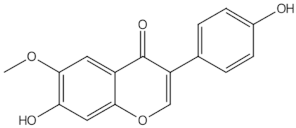
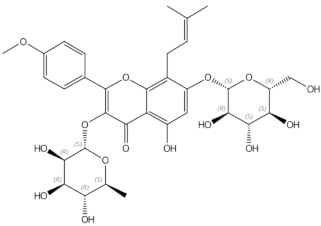
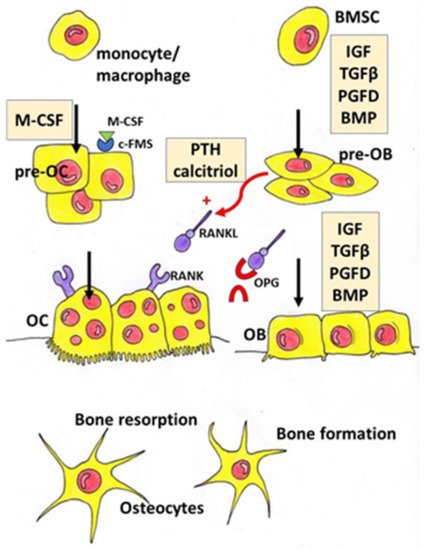
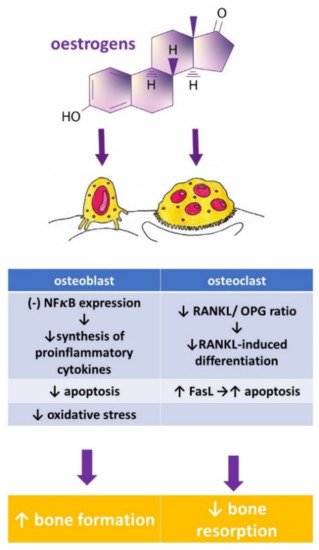
Osteoporosis is a systemic bone disease characterized by reduced bone mass and the deterioration of bone microarchitecture leading to bone fragility and an increased risk of fractures. Conventional anti-osteoporotic pharmaceutics are effective in the treatment and prophylaxis of osteoporosis, however they are associated with various side effects that push many women into seeking botanicals as an alternative therapy.
- osteoporosis
- menopause
- botanicals
- herbs
1. Introduction
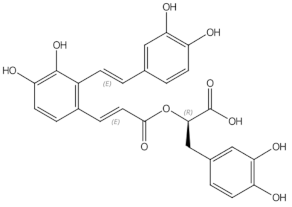
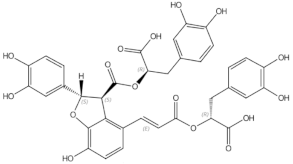
Herbal Compounds | Subgroup | Chemical Structure | Proposed Mechanism of Action | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
Daidzein | isoflavones | | ER mediated signalling pathway, activation of intracellular pathways: AKT, phospholipase C (PLC), mitogen-activated protein kinase (MAPK) [14 | ||||||||
eudesmane-type sesquiterpenoid | |||||||||||
| |||||||||||
| |||||||||||
Osteoclastogenesis inhibition and ovariectomy-induced bone loss prevention by regulating RANKL-Induced NF-κB, c-Fos and Calcium Signaling | |||||||||||
[ | ] |
AKT—protein kinase B, BALP—bone-specific alkaline phosphatase, CTX—type I collagen cross-linked C-telopeptide, DPD—deoxypyridinoline, ER—oestrogen receptor, ERK—extracellular signal-regulated kinase, JNK—c-Jun N-terminal kinase, MAPK—mitogen-activated protein kinase, NFκB—nuclear factor-kappa B, NTX—type I collagen cross-linked N-telopeptide, PLC—phospholipase C, RANKL—Receptor Activator for Nuclear Factor κB Ligand, TRAP 5b—Tartrate-resistant acid phosphatase 5b.
Botanicals | Population and Design | Intervention | Outcome | Authors and References | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
] | |||||||||||||||||
Soy isoflavones | single open-group prospective clinical intervention; 42 postmenopausal women, | three daily servings for 12 consecutive weeks of whole soy foods containing approximately 60 mg/day of isoflavones | ↓ NTX, ↑ osteocalcin | Scheiber 2001 [27] | |||||||||||||
Genistein | isoflavones | | ER-mediated signalling pathway, activation of intracellular pathways: AKT, PLC, MAPK [14] | ||||||||||||||
Ipriflavone | isoflavones | | Modulation of key signalling pathways to regulate bone resorption (e.g., ↓urinary DPD, NTX) and bone formation (e.g., ↑BALP and osteocalcin [15] | ||||||||||||||
Biochanin A | O-methylated isoflavones | | ER mediated signalling pathway, activation of intracellular pathways: AKT, PLC, MAPK [14] | ||||||||||||||
] | Formononetin | O-methylated isoflavones |
| ||||||||||||||
prenylflavonoids | | Promotion of osteoblast differentiation and induction of osteoclast apoptosis [20] | |||||||||||||||
Epimedin B | prenylflavonoids | | Inhibition of bone resorption, bone formation promotion and urinary calcium excretion blocking [21] | ||||||||||||||
Epimedin C | prenylflavonoids | | Inhibition of bone resorption, bone formation promotion and urinary calcium excretion blocking [21] | ||||||||||||||
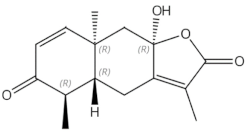
Tanshinones (dihydrotanshinone, tanshinone I, or tanshinone IIA) | diterpenes | | Inhibition of the TRAP5b-expressing osteoclasts formation by suppressing RANKL-induced expression of c-fos and NFATc1 [22 | ||||||||||||||
Soy isoflavones | RCT with 3 groups: soy rich diet, HRT, control; 187 healthy asymptomatic postmenopausal women aged 39–60, | approximately 47 mg/day of isoflavones in diet group; duration: 6 moths | ↑ bone osteoblastic activity but not as effective as HRT in reducing the postmenopausal turnover, ↑ osteocalcin | Chiechi 2002 [28] | |||||||||||||
Soy isoflavones | RCT with 3 groups: placebo, mid-dose, and | high-dose, in pill form; 203 postmenopausal Chinese women aged 48 to 62, | placebo (daily dose of 0 mg isoflavones + 500 mg calcium, n = 67) mid-dose (40 mg isoflavones + 500 mg calcium, n = 68) and high-dose (80 mg isoflavones + 500 mg calcium, n = 68); duration: 12months | favourable effect on rates of change in BMC at the total hip and trochanter among later postmenopausal women (>4 y), in women with lower body weight (≤median, 55.5 kg), or among women with lower level of calcium intake (≤median, 1095 mg/day) | Chen 2004 [29] | ||||||||||||
Soy isoflavones | RCT with 3 groups: placebo, mid-dose, and | high-dose; 90 Chinese postmenopausal women aged 45–60 | placebo (daily dose of 0 mg isoflavones) mid-dose (84 mg) and high dose (126 mg), 30 subjects/group; duration: 6months | Retardation of lumbar and femoral bone loss at the lumbar spine (L1–L4) and bone resorption | Ye 2006 [ | ||||||||||||
Soy isoflavones | double-blind RCT with 2 groups: placebo, isoflavone conjugates in capsule form, 68 postmenopausal Japanese women | ER mediated signalling pathway, activation of intracellular pathways: AKT, PLC, MAPK |
Isoflavone group (75 mg of isoflavone conjugates/day), 34 subjects/group; duration: 12 months |
[14] |
|||||||||||||
↑ serum equol in the equol producers but not in the nonproducers, preventive effects of isoflavones on hip BMD | Wu 2007 [31] | Glycitein | O-methylated isoflavones | ||||||||||||||
Soy isoflavones | double-blind RCT with 3 groups: placebo, mid-dose, and | | high-dose in tablet form; 255 postmenopausal women aged 46–63 | ||||||||||||||
Dried plums | |||||||||||||||||
RCT with 3 groups: placebo, 2 dose of dried plums, 48 older postmenopausal women | |||||||||||||||||
control (0 g/day dried plum), dried plum (50 or 100 g/day | dried plum), duration: 6 months | ↑ BMD, ↓ TRAP-5b, ↑ BALP/TRAP-5b ratio | Hooshmand 2016 [55] | ||||||||||||||
Dried plums | RCT with 3 groups: placebo, 2 dose of dried plums; 35 men between the ages of 55 and 80 with moderate bone loss | control group (0g prunes), 100 g prunes daily, 50 g prunes daily, + multivitamin containing 450 mg calcium and 800 IU vitamin D for all group, duration: 3 months | ↓ osteocalcin, ↑ OPG/RANKL ratio | Ajmandi 2020 [56] | |||||||||||||
Horsetail (Equisetum arvense) | Double blind RCT with 4 groups: control, placebo + horsetail extract, horsetail extract, calcium, 122 women in menopause for at least two years | no treatment/control group (n = 29), placebo for 40 days and titrated horsetail extract for a further 40 days (n = 31), titrated dry horsetail extract for 80 days (n = 30); Calcium (Osteosil®) for 80 days (n = 32), After the 80-day initial study period, patients treated with titrated horsetail extract and with calcium continued treatment for one year | ↑ in the average densitometric values for the vertebra | Corletto 1999 [57] | |||||||||||||
Black cohosh (Cimcifuga racemosa) | double-blind RCT with 3 groups: placebo, black cohosh, oestrogens; 62 postmenopausal women | placebo, black cohosh (40 mg of herbal drug/day), conjugated oestrogens (0.6 mg/day); duration: 12 weeks. | ↑ osteoblast activity, weak estrogen-like activity, no significant effects on coagulation markers and liver enzymes | Wuttke 2006 [58] | |||||||||||||
Black cohosh (Cimcifuga racemosa) | prospective clinical trial with 2 groups: untreated control, isopropanolic extract of Cimicifuga racemosa, 82 postmenopausal women | control group (n = 37), isopropanolic extract of Cimicifuga racemosa (Remifemin®, 40 mg/day, n = 45), duration: 3 months | ↓NTX (marker of bone resorption), ↑ ALP (marker of bone formation) | Garcia-Pérez 2009 [59] | |||||||||||||
Black cohosh (Cimcifuga racemosa) | RCT with 3 groups: control (CG), exercise group (EG), exercise and Cimicifuga racemosa (CR) supplementation group (EGCR), 128 early postmenopausal women | CG (wellness control, n = 42), EG (n = 43), EGCR (40 mg/day of CR BNO 1055; n = 43), Calcium (1500 mg/d) + vitamin D (500 IE/d) supplementation for all participant duration:12 months | CR (CR BNO 1055) did not enhance positive effects of exercise on BMD at the lumbar spine | Bebenek 2010 [60] | |||||||||||||
Labisia pumila and Eurycoma longifolia | double-blind RCT with 2 groups: placebo, Nu-femme™, 119 healthy women aged 41–55 years experiencing peri-menopausal or menopausal symptoms | placebo (n = 59), herbal formulation (Nu-femme™, n = 60) = 200mg Labisia pumila (SLP+®) + 50mg Eurycoma longifolia (Physta®); duration: 24 weeks | No significant effect on bone formation (BALP) and resorption (NTX) markers | Chinnappan 2020 [61] |
ALP—alkaline phosphatase, BALP—bone-specific alkaline phosphatase, BMC—bone mineral content, BMD—bone mineral density, CTX—type I collagen crosslinked beta C-telopeptide, DPD—deoxypyridinoline, HRT—hormonal replacement therapy, IGF-1– insulin-like growth factor 1, NTX—type I collagen crosslinked N- telopeptide, OPG—osteoprotegerin, P1NP—type I procollagen-N-propeptide, RANKL—Receptor Activator for Nuclear Factor κB Ligand, SSI—strength strain index, ↑—increased, ↓—decreased
2. Phytoestrogens
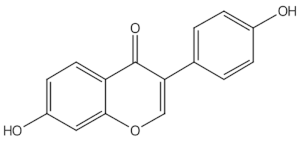
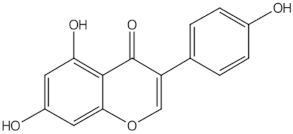
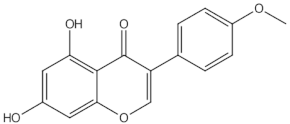
2.1. Isoflavones
Aglycones | Glycosides | Acetylglycosides | Malonyl Isoflavone Glycosides | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Daidzein | Daidzin | Acetyldaidzin | Malonyldaidzin | |||||||||||||||
Genistein | Genistin | Acetylgenistin | Malonylgenistin | |||||||||||||||
Glycitein | Glycitin | Acetylglycitin | Malonylglycitin | |||||||||||||||
Biochanin A | Sissostrin | Malonylsissostrin | ||||||||||||||||
Formononetin | Ononin | Malonylononin | ||||||||||||||||
placebo (daily dose of 0 mg isoflavones) mid-dose (80 mg) and high dose (120 mg); duration: 3 years | ER mediated signalling pathway, activation of intracellular pathways: AKT, PLC, MAPK [14] | |||||||||||||||||
mild beneficial femoral BMD—and SSI | ||||||||||||||||||
Daidzein | Shedd-Wise 2011 | [32] |
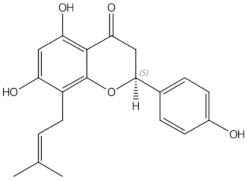
Daidzin | Icariin | prenylated flavonol glycoside | |||||||||||||
Acetyldaidzin | Malonyldaidzin |
Soy isoflavones | double-blind RCT with 2 groups: placebo, isoflavones in tablet form; 87 Korean postmenopausal women aged 45–60 | | Isoflavone group = 70 mg in 2 tablet per day (8.0 mg glycitin, 20 mg daidzein, and 12.4 mg genistin); duration: 12 weeks | Stimulation of bone formation by promotion of osteoblasts differentiation and enhancement of their activity [16]; activation of BMP-2/Smad4, Wnt and IGF-1 signal transduction pathways [5][5[17],17], induction of ERK, JNK and p38 kinase activation [18]; decreasing of RANKL-induced osteoclastogenesis via inhibition of NFκB and MAPK expression [19] | ||||||||||||
↑ serum BALP and osteocalcin | Lee 2017 [33] | 8-prenylnaringenin | ||||||||||||||||
Soy isoflavones | RCT with 3 groups; placebo, HRT, phytoestrogens; 325 postmenopausal women | HRT group (1 mg oestradiol and 0.5 mg norethisterone acetate p.o. daily, phytoestrogens group (40% standardized extract with 20 mg soy isoflavones (genistein and daidzein), two capsules = 40 mg p.o. daily; duration: 12 months | no significant differences between the effectiveness of the HRT and phytoestrogen in terms of effects on BMD and bone resorption | Tit 2018 [34] | ||||||||||||||
Soy isoflavones | double-blind RCT with 3 groups: placebo, soy protein, soy protein + isoflavone in snack bar; 200 women within 2 years of the onset of their menopause | placebo (isoflavone of less than 300 parts per billion) PI (15 g soy protein with 66 mg of isoflavones), SP (15 g soy protein alone, isoflavone free) daily, 100 women/group; | duration: 6 months | ↓CTX with SPI supplementation compared to SP, ↓ P1NP with SPI supplementation | Sathyapalan 2017 [35] | |||||||||||||
Soy isoflavones | double-blind RCT with 2 groups: placebo, isoflavones in form of tablet | placebo (0 mg of isoflavones), isoflavones extracted from soy protein (200 mg daily = 4tablets) 248 multi-ethnic menopausal women aged 45 to 60; duration: 2 years | not superior to placebo in preventing bone loss or in reducing bone turnover or menopausal symptoms in women in the first 5 years of menopause | Levis 2011 [36] | ] | |||||||||||||
Soy isoflavones | double-blind RCT with 2 groups: placebo, phytoestrogens; 202 postmenopausal women aged 60–75 | [ |
placebo (milk protein), phytoestrogens (25.6 g soy protein containing 52 mg genistein, 41 mg daidzein and 6 mg glycetein (aglycone weights; duration: 12 months | 23] | ||||||||||||||
no significant differences for BALP, calcium, and phosphorus measurements. | Kreijkamp-Kaspers 2004 [37] | Salvianolic acid A | phenolic acids | |||||||||||||||
Soy isoflavones | | osteoblast differentiation modulation and osteoblast activity upregulation [24 |
double-blind, multicentre RCT with 2 groups: isoflavone-enriched biscuits and bars and control biscuits and bars; 237 early postmenopausal women aged 53 ± 3y | ][25 |
placebo group (biscuits and cereal bar), isoflavone- enriched foods (soy isoflavone concentrate containing 40–50% of isoflavones) providing a mean daily intake of 110 mg isoflavone aglycones/day; duration: 12 months] | |||||||||||||
isoflavone-enriched products did not alter lumbar and total body BMD or markers of bone formation and bone resorption | Brink 2008 [38] | Salvianolic acid B | phenolic acids | | ||||||||||||||
Genistein | double-blind RCT with 2 groups: placebo, genistein; | 389 postmenopausal women | osteoblast differentiation modulation and osteoblast activity upregulation |
placebo group (calcium and vitamin D, n = 191), genistein aglycone group (54 mg/day + calcium and vitamin D, n = 198) | duration: 36 months | |||||||||||||
↑lumbar and femoral BMD, ↓bone resorption markers (DPD, CTX, RANKL), ↑ bone formation markers (BALP, IGF-1 and OPG) | Eudebeiolide B | |||||||||||||||||
Genistein | double-blind RCT with 2 groups: placebo, genistein; | 138 postmenopausal women (age 49–67 years) | placebo (0mg of isoflavones, n = 67), genistein (54 mg/day, n = 71), duration: 24 months | ↑ femoral and lumbar BMD, improvement of the quantitative ultrasound parameters (stiffness index, amplitude-dependent speed of sound, and bone transmission time) | Atteritano 2009 [41] | |||||||||||||
Genistein | double-blind RCT with 2 groups: placebo, geniVida™ bone blend group; 70 postmenopausal women | placebo (calcium only, n = 28), genistein group = 30 mg/daygenistein + vitamin D3 (800 IU/days) + vitamin K1 (150 μg/days) + polyunsaturated fatty acids (1 g polyunsaturated fatty acids as ethyl ester: eicosapentaenoic acid/docosahexaenoic acid ratio = ~2/1, n = 30); | duration: 6 months | ↑ BMD, ↑ BALP and NTX | Lappe 2013 [42] | |||||||||||||
Genistein | double-blind RCT with 2 groups: placebo, genistein, 121 postmenopausal women | placebo (1000 mg of calcium and 800 IU vitamin D3; n = 59), genistein aglycone group (54 mg/day + calcium, vitamin D3; n = 62, duration: 24 months | ↑ femoral and lumbar BMD, ↑ BALP | Arcoraci 2017 [43] | ||||||||||||||
Red clover isoflavones (genistein, daidzein, formononetin, biochanin A) | double-blind RCT with 4 groups: placebo, red clover isoflavone preparation (Rimostil) in 3 doses, 46 postmenopausal women | placebo, Rimostil (phytoestrogens)—28.5 mg, 57 mg, or 85.5mg/day, duration: 6 months, | ↑ BMD after 57 mg and 85.5 mg/day | Clifton-Bligh 2001 [44] | ||||||||||||||
Red clover isoflavones | double-blind RCT with 2 groups: placebo, isoflavone supplement Promensil®; 205 pre-, peri-, and postmenopausal women aged 49–65 | placebo, isoflavone supplement (providing 26 mg biochanin A, 16 mg formononetin, 1 mg genistein, 0.5 mg daidzein/daily); duration: 12 months | ↑ bone formation markers (BALP, P1NP), ↓ lumbar spine BMC and BMD | Akinson 2004 [45] | ||||||||||||||
Red clover isoflavones | double-blind, parallel RCT with 2 groups: placebo, red clover extract; 78 postmenopausal osteopenic women supplemented with calcium 1200 mg/day, magnesium 550 mg/day, calcitriol 25 µg/day | placebo, red clover extract (60 mg isoflavone aglycones/day + probiotics); duration: 12 months | ↓ lumbar and femoral BMD loss, ↓ CTX | Lambert 2017 [46] | ||||||||||||||
Red clover isoflavones | double-blind RCT with 2 groups: placebo, red clover extract; 60 menopausal women | placebo, red clover extract (daily dose of 150 mL containing 37.1 mg isoflavones = 33.8 mg as aglycones); duration: 12 weeks | ↑ spinal BMD | Thorup 2015 [47] | ||||||||||||||
Red clover isoflavones | double-blind RCT with 2 groups: placebo, standardized red clover isoflavone dietary supplement (Promensil®); 401 healthy women aged 35–70 years | Placebo, red clover isoflavones (40 mg/day); | duration: 36 months | safe and well tolerated but no effect on BMD | Powles 2008 [48] | |||||||||||||
Red clover isoflavones | double-blind RCT with 3 groups: placebo and 2 dietary supplements derived from red clover, 252 menopausal women ages 45–60 years | placebo, Promensil® (82 mg total isoflavones), Rimostil® (57.2 mg total isoflavones), duration: 12 weeks | no effect on bone turnover markers. | Knudson Schult 2004 [49] | ||||||||||||||
Kudzu root (Pueraria candollei var. mirifica) | double-blind RCT with 4 groups: placebo, 3 dose of Pueraria; 71 postmenopausal women aged 45 to 60 years | placebo (n = 20), Pueraria mirifica in capsules (20, 30, or 50 mg once daily, n = 51); duration: 24 weeks | ↓ BALP | Manonai 2008 [50] | ||||||||||||||
Kudzu root (Pueraria candollei var. mirifica) | double-blind RCT with 2 groups 19 postmenopausal women | placebo tablet, tablet containing 25 mg dried PM root powder, 4 tablets/day; duration: 2 months | ↓ ALP | Okamura 2008 [51] | ||||||||||||||
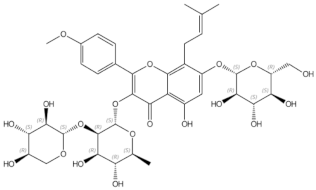
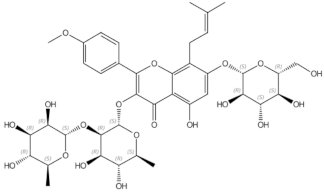
Epimedium | double-blind RCT with 2 groups: placebo, Epimedium-derived phytoestrogen flavonoids (EPF), 100 healthy late postmenopausal women | placebo (n = 50), EPF group (n = 50; a daily dose of 60 mg Icariin, 15 mg daidzein, and 3 mg genistein), +300 mg calcium daily for both group; duration: 24 months | ↑ lumbar and femoral BMD, ↓ DPD, | Zang 2007 [52] | ||||||||||||||
Dried plums | RCT with 2 groups: placebo (dried apples), dried plums; 58 postmenopausal women | placebo (dried apples 75 g daily), dried plums (100 g daily); duration: 3 months | ↑IGF-1, ↑ ALP, ↑ BALP | Ajamandi 2002 [53] | ||||||||||||||
Dried plums | RCT with 2 groups: placebo, dried plums, 160 | postmenopausal women | with osteopenia | placebo (dried apples 75 g daily), dried plums (100 g daily) + 500 mg Calcium, 400 IU (10 μg) vitamin D daily for both group; duration: 12 months | ↑ ulnar and lumbar BMD, ↓ BALP | Hooshmand 2011 [54] |