Helicobacter pylori infection still remains one of the most prevalent infections worldwide, especially in low-resource countries, and the major risk factor for peptic ulcer and gastric cancer. The "test-and-treat" strategy is recommended by several guidelines and consensus. The choice of testing method is based on patient age, presence of alarm signs and/or symptoms, use of non-steroidal anti-inflammatory drugs, as well as local availability, test reliability and cost.
Culture is the gold standard to detect H. pylori and, possibly, to perform susceptibility testing, however, it requires upper endoscopy and dedicate labs. Recent advances in molecular biology provide new strategies in detecting the infection and antimicrobial resistance without invasive tests.
- Helicobacter pylori
- testing
- antibiotic resistance
- molecular techniques
- artificial intelligence
1. [1][2][3][4][5][6][7][8][9][10][11][12][13][14][15][16][17][18][19][20][21][22][23][24][25][26][27][28][29][30][31][32][33][34][35][36][37][38][39][40][41][42][43][44][45][46][47][48][49][50][51][52][53][54][55][56][57][58][59][60][61][62][63][64][65][66][67][68][69][70][71][72][73][74][75][76][77][78][79][80][81][82][83][84][85][86][87][88][89][90][91][92][93][94]Helicobacter pylori infection
The infection can be essentially detected by invasive and non-invasive tests. The choice of technique relies upon the patient needs. Presence of alarm symptoms, use of non-steroidal anti-inflammatory drugs (NSAIDs), advanced age (>45-50, years or >60 years) [1][2][3][4][1-4] history of premalignant conditions or follow up for a previous malignant disease dictates an upper endoscopy evaluation. The indication for esophago-gastric-duodenoscopy allows to directly observe the mucosa, to collect biopsy samples for histology examination, urease test, bacterial culture and molecular assay. In the absence of endoscopy recommendation, non-invasive tests such as urea breath testing or stool antigen assay are appropriate to confirm an active infection. Serology may be used in specific setting to assist the physician in the diagnosis of bacterial infection [5]. However, the diagnostic strategy cannot prescind from the local availability, costs of the test, labs reliability and patient preferences. As a rule of thumb, it is important for the physician to confirm the diagnosis, to evaluate the presence of gastric lesions induced by the infection according to the patient clinical history, to offer H. pylori eradication therapy and to check treatment success.
2. Invasive Tests
2.1.Invasive Endotestscopy
Endoscopy
For patients in whom an upper endoscopy is required, where available, advanced endoscopy techniques should be preferred to conventional endoscopy, especially for patients/subjects with a high pretest probability to harbor premalignant lesions, such as those from countries or subpopulations with high prevalence of gastric cancer, or individuals with strong familiarity for gastric malignancy or those patients who need a strict endoscopy surveillance for previous diagnosed premalignant lesions.
By standard white light endoscopy (WLE) the infection may be identified on the base of specific gastric mucosa features such as the presence of antral nodularity with a sensitivity and a specificity ranging from 39.8% to 96.4% and from 83.6% to 100%, respectively [6]. Additional reports identified the erythema, erosions, thickened folds or absence of rugae, mosaic appearance with or without hyperemia and visible submucosal vessels in the gastric mucosa as the hallmarks of H. pylori infection [7][8][9][10][7-10] or gastric black spots associated with H. pylori eradication [11], and mucosal swelling (77%) associated with mild atrophy [12]. However, the low interobserver agreement may be a limitation to translate gastric mucosal features into a diagnosis of specific gastritis with or without H. pylori infection.
The results obtained with the narrow band imaging (NBI), which uses blue light from a laser source (415 nm) to highlight the vascular architecture of the gastric mucosa are more promising. Based on distinct patterns of the gastric mucosa the endoscopist may predict H. pylori infection by conventional NBI [13] and by the magnifying NBI technique the presence of intestinal metaplasia with a sensitivity and specificity greater than 95% [14]. Moreover, a high degree of concordance was observed between magnifying NBI and the operative link for gastritis and for gastric intestinal metaplasia assessment [15][16][15,16]. Interestingly, by this technique, specific morphological patterns, including reddish depressed lesions, are frequently observed in association with H. pylori eradication [14][17][14,17]. The magnifying endoscopy with NBI proved to be superior to WLE and chromoendoscopy in the diagnosis of early gastric cancer after H. pylori eradication [18]. The confocal laser endomicroscopy is more accurate than NBI for grading gastric premalignant lesions [19]. The blue laser imaging (BLI) and the linked color imaging (LCI) are also highly accurate in detecting H. pylori infection and premalignant lesions related to the infection [20][21][22][23][24][25][20-25]. Endocytoscopy (EC), an ultra-high magnification endoscopy, is able to provide histologic assessment in vivo [26]. Overall, high-definition endoscopy allows in real time the diagnosis of H. pylori infection, detection of premalignant and malignant gastric lesions and targeted mucosa biopsy sampling.
All recent developments of high-definition endoscopy for the diagnosis of H. pylori infection and detection of pre-malignant and malignant gastric lesions, allowing a real-time decision-making, prompted the revision of Kyoto endoscopic classification [27].
In the last years there was also an attempt to use more sophisticated tools to diagnose H. pylori by using artificial intelligence approach mimicking the brain neural network [28]. In a recent meta-analysis, the artificial intelligence algorithm demonstrated to be an accurate tool for the prediction of H. pylori infection during endoscopic procedures, although the real application needs to be evaluated in clinical studies [29].
2.2. Histology
Histology
The examination of gastric mucosal biopsy specimens remains the gold standard for detection of H. pylori, with a sensitivity of 95% and a specificity of 98%. In addition, it enables the visualization of gastric morphology at any time. In order to obtain an accurate diagnosis, two antral biopsies including one from the gastric angulus, and two biopsies from the corpus are necessary [30]. The widespread use of proton pump inhibitors (PPIs) may result in atypical presentation of gastritis or in density variation of bacteria at different sites [31], the accuracy of histologic diagnosis of H. pylori infection can be improved by using special staining techniques, specific immune stain or digital pathology [32][33][32,33]. Gastric biopsy specimens obtained by high-definition or conventional endoscopy can be used for molecular testing to assess the presence of H. pylori and its antibiotic susceptibility profile also in patients under PPI treatment. This is particularly useful for those patients who cannot stop the PPI treatment (for instance because of double antiplatelet treatments, or with a Zollinger Ellison syndrome or similar circumstances).
2.3. Rapid Urease Test
Rapid Urease Test
Upper endoscopy enables to collect also biopsy specimens for urease testing. The method is based on the presence of pre-formed urease by the bacteria and, in media containing urea, the enzyme releases ammonia increasing the pH and resulting in a color change of the medium.
The urease test is rapid (RUT), easy to perform, highly specific and inexpensive for H. pylori diagnosis, although it requires at least a 104 bacterial load in the gastric specimens [34]. False-negative results may occur with recent use of antibiotics, bismuth-containing compounds, PPIs, especially omeprazole and lansoprazole, and in children younger than five years [35]. To collect biopsies from the corpus rather than from the antrum, or combining antral and corpus biopsies has demonstrate to enhance RUT sensitivity [36][37][36,37]. In addition to false negative, also false-positive RUT may occur in presence of urease positive bacteria [34]. The gastric samples used for RUT can be re-used for molecular testing in order to identify bacterial resistance. However, compared with histology, RUT does not allow to plan a correct follow up for the patient.
2.4. Culture
Culture
In addition to histological examination and rapid urease testing, upper endoscopy offers the opportunity to collect gastric specimens for bacterial culture, susceptibility testing and even organism genotyping. Although culture is highly specific, it has a low sensitivity as H. pylori is difficult to grow and experienced laboratories are required. Sensitivity may be improved by sending the specimen to the laboratory within 30 minutes from collection, using a warm and non-selective culture medium, a longer incubation and the addition of hydrogen in the atmosphere, or by treating specimens with trypsin [38][39][40][41][38-41].
3. Non-invasive tests
Non-invasive tests can be divided into those able to detect an active infection, such as the urea breath test and stool antigen test, and those able to provide information on current or prior H. pylori infection without discrimination.
3.1. Urea breath test
Urea breath test
The 13C-urea breath test (UBT) is the non-invasive method of choice to determine H. pylori status when available. Similarly to RUT, the test takes advantage from the urease produced by the bacteria, which hydrolyzes urea generating CO2 and ammonia. The urea substrate is enriched with a labeled carbon isotope that may be non-radioactive (13C) or radioactive (14C) and ingested, usually, with a test meal to prolong the permanence of urea in the stomach. Breath exhaled samples are collected in proper tubes before and after urea ingestion. Even though the dose of radiation is small in the 14C-UBT, the non-radioactive 13C test is routinely preferred. The test is also used to ascertain the eradication and it is recommended for the “test-and-treat” strategy in dyspeptic patients [1]. The test could also be successfully applied to patients with partial gastrectomy, especially when performed with the patient in the right position [42]. The 13C-UBT shows high sensitivity (95%) and specificity (95% to 100%) [43].
The 13C-urea is available in the market in different formulations such as a powder, capsules and tablets ranging between 50 and 100 mg. The test meal containing citric acid or malic acid enhance 13C-UBT performance increasing urease activity in the presence of bacteria [44]. However, quantitative results may be influenced by sex, age, body mass index, especially obesity, smoking, gastric atrophy and intestinal metaplasia and even by the socioeconomic status [45][46][47][45-47]. The most used cutoffs, expressed as delta over baseline (DOB), are 2‰, 2.4‰, 2.5‰ and 5‰ [48]. To analyze labeled 13CO2 several detector devices are available in the market [49][50][49,50].
3.2. Stool antigen test
Stool antigen test
To culture H. pylori from feces is very difficult and time consuming [51], on the contrary non-invasive tests able to detect H. pylori antigen in stool specimens are simple to perform and large head-to-head comparisons with other tests demonstrated high diagnostic accuracy of this approach [52]. Nowadays several assays are available, the more recent ones are listed in table 1.
Table 1. Most recent stool antigen tests and their reported sensitivity and specificity.
|
Brand |
Based on |
Sensitivity |
Specificity |
Reference |
|
|
LIAISON H. pylori SA assay (DiaSorin, Saluggia, Italy) |
chemiluminescent immunoassay |
90.1 95.5
|
92.4 97.6 |
Ramirez-Lazaro et al., 2016 [53] |
|
|
Genx H. pylori card test (Genx Bioresearch, Kocaeli, Turkey) |
monoclonal immunochromatographic assay |
51.6 |
96.0 |
Korkmaz et al., 2015 [54] |
|
|
Uni-Gold™ H. pylori Antigen (Trinity Biotech, Bray, Ireland) |
monoclonal lateral flow immunochromatographic assays |
83.2 |
87-89.3 |
Lario et al., 2016 [55] |
|
|
RAPID Hp StAR (Oxoid Ltd., UK) |
monoclonal lateral flow immunochromatographic assays |
94-95 |
77.1 to 84.7 |
Lario et al., 2016 [55] |
|
|
ImmunoCard STAT! HpSA (Meridian Diagnostics, USA) |
monoclonal lateral flow immunochromatographic assays |
79-81.5 |
90.8-91.6 |
Lario et al., 2016 [55] |
|
|
IDEIA HpStAR®; ThermoFisher Sc., Waltham, USA |
monoclonal antibodies and the ELISA technique |
Before Hp treatment 93.6 After Hp treatment 100 |
Before Hp treatment 100 After Hp treatment 92.8 |
Moubri et al., 2018 [56] |
|
|
Quick Chaser H. pylori®, QCP, Misuho Medy, Tosu, Japan) |
immunochromatography |
92.3 |
|
Kakiuchi et al., 2019 [57] |
|
|
Vstrip®HpSA, (Meridian), |
immunochromatography |
91% |
97% |
Fang et al., 2020 [58] |
|
|
ImmunoCard STAT!® Campy (Meridian) |
immunochromatography |
76.9% |
97% |
Fang et al., 2020 [58] |
|
|
Brand |
Based on |
Sensitivity |
Specificity |
Reference |
|
|
LIAISON H. pylori SA assay (DiaSorin, Saluggia, Italy) |
chemiluminescent immunoassay |
90.1 95.5
|
92.4 97.6 |
Ramirez-Lazaro et al., 2016 [53] |
|
|
Genx H. pylori card test (Genx Bioresearch, Kocaeli, Turkey) |
monoclonal immunochromatographic assay |
51.6 |
96.0 |
Korkmaz et al., 2015 [54] |
|
|
Uni-Gold™ H. pylori Antigen (Trinity Biotech, Bray, Ireland) |
monoclonal lateral flow immunochromatographic assays |
83.2 |
87-89.3 |
Lario et al., 2016 [55] |
|
|
RAPID Hp StAR (Oxoid Ltd., UK) |
monoclonal lateral flow immunochromatographic assays |
94-95 |
77.1 to 84.7 |
Lario et al., 2016 [55] |
|
|
ImmunoCard STAT! HpSA (Meridian Diagnostics, USA) |
monoclonal lateral flow immunochromatographic assays |
79-81.5 |
90.8-91.6 |
Lario et al., 2016 [55] |
|
|
IDEIA HpStAR®; ThermoFisher Sc., Waltham, USA |
monoclonal antibodies and the ELISA technique |
Before Hp treatment 93.6 After Hp treatment 100 |
Before Hp treatment 100 After Hp treatment 92.8 |
Moubri et al., 2018 [56] |
|
|
Quick Chaser H. pylori®, QCP, Misuho Medy, Tosu, Japan) |
immunochromatography |
92.3 |
|
Kakiuchi et al., 2019 [57] |
|
|
Vstrip®HpSA, (Meridian), |
immunochromatography |
91% |
97% |
Fang et al., 2020 [58] |
|
|
ImmunoCard STAT!® Campy (Meridian) |
immunochromatography |
76.9% |
97% |
Fang et al., 2020 [58] |
|
Overall, stool monoclonal antibody tests are superior to polyclonal antibody tests and demonstrated a pooled sensitivity and specificity of 93% and 96%, respectively [59][60][59,60]. They also show an excellent diagnostic accuracy in pediatric setting, especially when tests are ELISA based rather than immunochromatography based [61]. The use of stool antigen test (or UBT) for the initial diagnosis of H. pylori infection and post-treatment (when endoscopy is not required), is recommended by the majority of guidelines and consensus [1][2][3][4][1-4].
The advantage of the UBT and of stool antigen test is that they assess the overall content of the stomach whereas hystology and RUT test only the tiny biopsy specimen. Theoretically and practically, the UBT and stool antigen test are the best methods for detection of active H. pylori infection. However, any drug that will diminish H. pylori load below the detection threshold of can cause false negative tests, particularly recent use of PPIs, bismuth-containing compounds or antibiotics.
3.3. Molecular Testing
Molecular testing
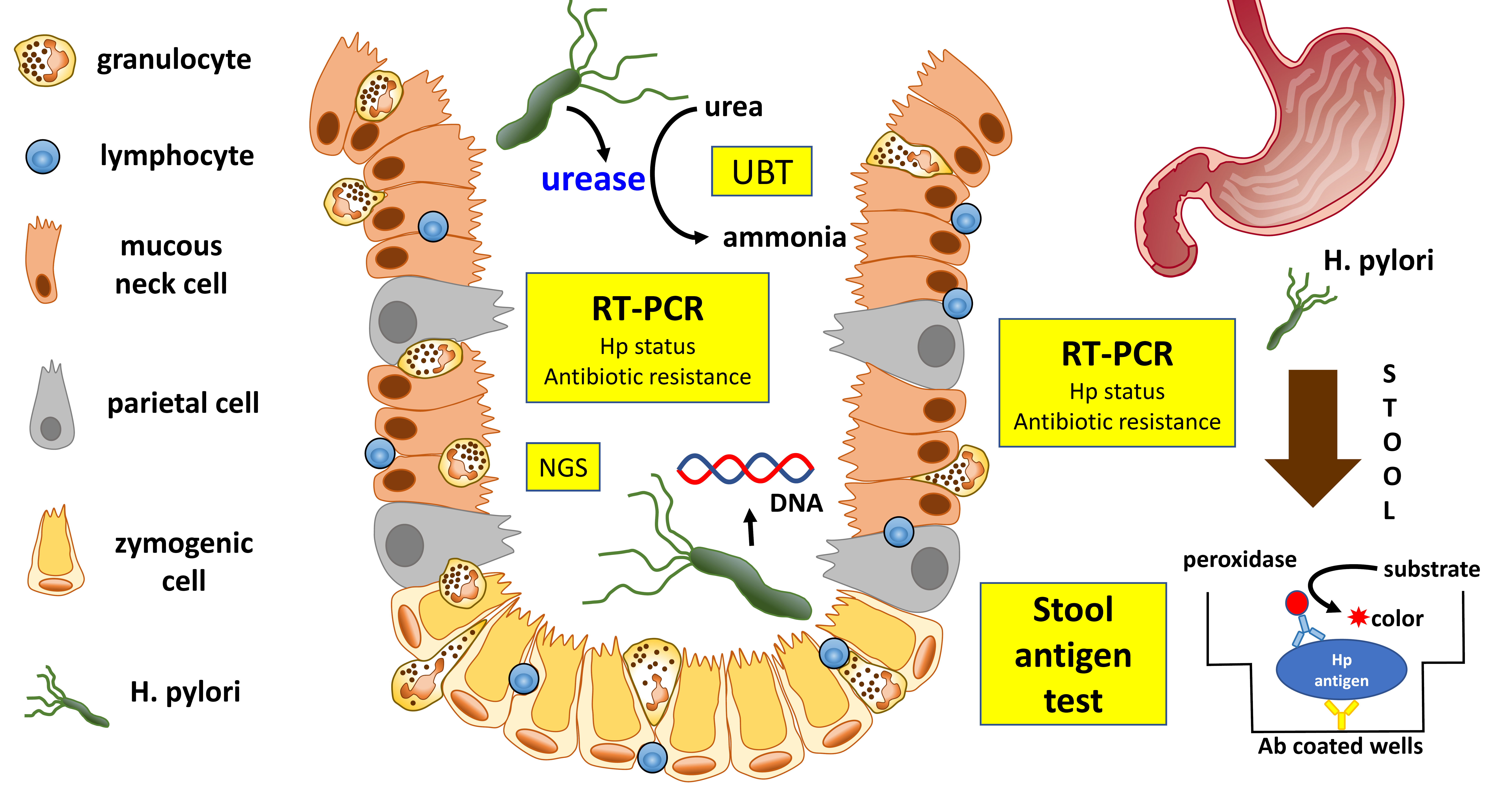
Molecular techniques should be preferred when available. The traditional or modified real-time (RT) PCR allows to detect the bacteria, and to screen for antibiotic sensitivity [62][63][64][62-64]. Moreover, real-time PCR is more accurate compared with other techniques for the detection of H. pylori in patients exposed to PPI [65], and is able to detect as low as 10 copies in adult [66] and children [67]. In addition to gastric biopsies, molecular testing can be applied to the gastric mucus present on biopsy forceps placed into water or into the RUT gel [68]. Alternatively, molecular tests to detect H. pylori and its susceptibility to antibiotics can be performed on gastric juice [69][70][71][69-71]. A droplet-digital PCR may also be applied to formalin-fixed, paraffin-embedded gastric tissue to determine the presence of clarithromycin resistance [72] or by next generation sequencing to determine levofloxacin and tetracycline resistance [73] (Figure 1).
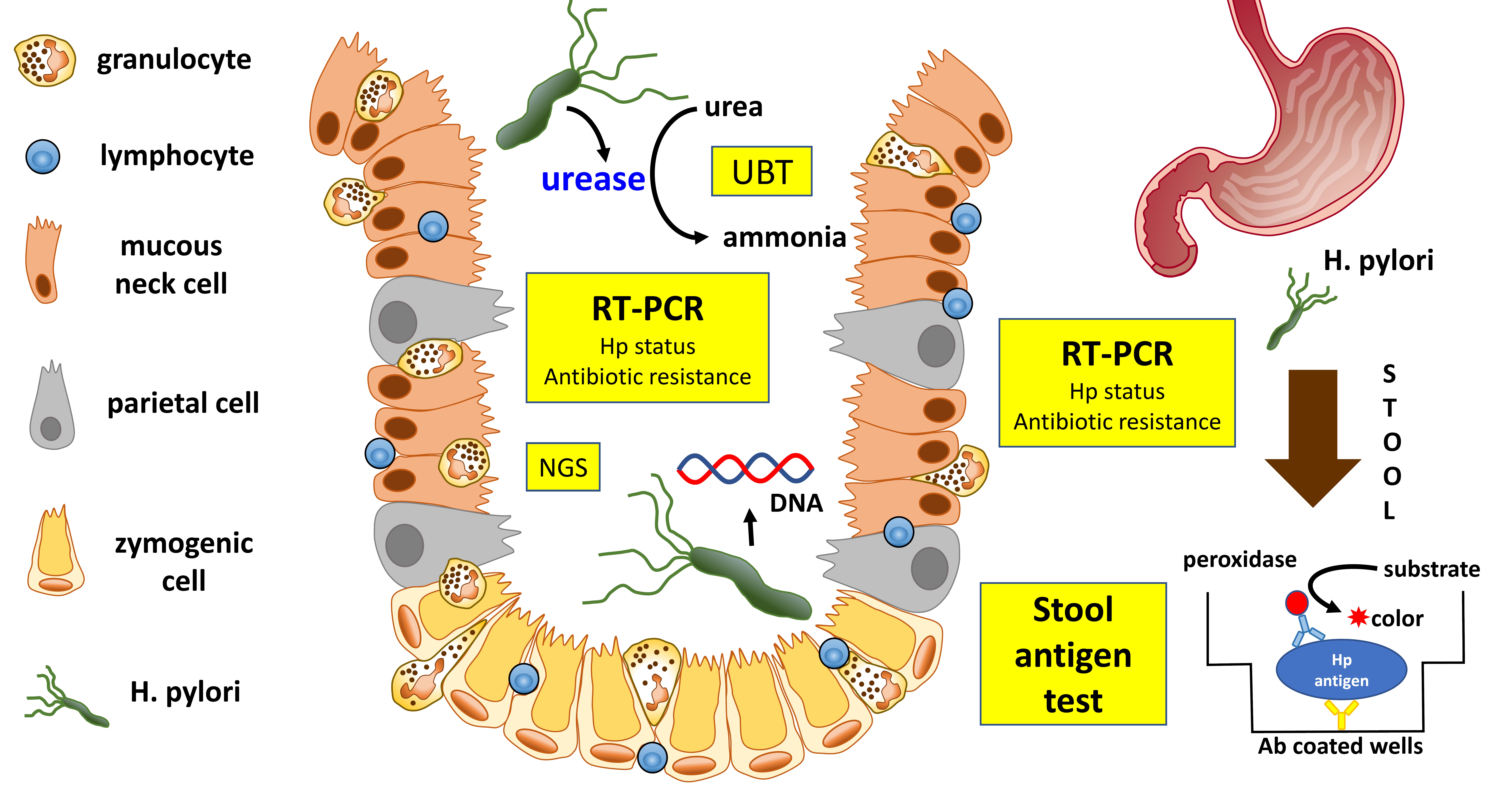
Figure 1. Invasive, non-invasive methods and molecular testing to detect H. pylori and its antibiotic resistance.
Invasive, non-invasive methods and molecular testing to detect H. pylori and its antibiotic resistance

Several molecular tests have been developed in the last years to detect specific H. pylori antigens and/or resistance pattern in the stool (Table 2).
Table 2. Recent molecular assays available to detected H. pylori and its antibiotic resistance.
Molecular test |
H. pylori DNA target |
Reference |
|
multiple genetic analysis system (MGAS) |
16S rDNA and ureC |
Zhou et al., 2015 [74] |
|
allele-specific PCR |
N87I mutation in the gyrA |
Trespalacios et al., 2015 [75] |
|
droplet-digital PCR (ddPCR) |
cagA and its EPIYA phosphorylation motifs |
Talarico et al., 2016 [64] |
|
loop-mediated isothermal amplification (LAMP) |
ureC gene |
Yari et al., 2016 [76] |
|
TaqMan RT-PCR |
A2142C, A2142G and A2143G mutations |
Beckman et al., 2017 [77] |
|
droplet-digital PCR (ddPCR)
|
16S rDNA |
Talarico et al., 2018 [72] |
|
real-time PCR (THD fecal test®) |
23S ribosomal RNA |
Iannone et al., 2018 [78] |
|
MagNA Pure 96 (Roche) |
DNA |
Clines et al., 2019 [79] |
|
Amplidiag® H. pylori + ClariR |
H. pylori and CLA resistance mutations |
Pichon et al., 2020 [80] |
|
Molecular test |
H. pylori DNA target |
Reference |
|
multiple genetic analysis system (MGAS) |
16S rDNA and ureC |
Zhou et al., 2015 [74] |
|
allele-specific PCR |
N87I mutation in the gyrA |
Trespalacios et al., 2015 [75] |
|
droplet-digital PCR (ddPCR) |
cagA and its EPIYA phosphorylation motifs |
Talarico et al., 2016 [64] |
|
loop-mediated isothermal amplification (LAMP) |
ureC gene |
Yari et al., 2016 [76] |
|
TaqMan RT-PCR |
A2142C, A2142G and A2143G mutations |
Beckman et al., 2017 [77] |
|
droplet-digital PCR (ddPCR)
|
16S rDNA |
Talarico et al., 2018 [72] |
|
real-time PCR (THD fecal test®) |
23S ribosomal RNA |
Iannone et al., 2018 [78] |
|
MagNA Pure 96 (Roche) |
DNA |
Clines et al., 2019 [79] |
|
Amplidiag® H. pylori + ClariR |
H. pylori and CLA resistance mutations |
Pichon et al., 2020 [80] |
3.4. Serology
Serology
Unlike UBT and stool antigen testing, serology does not distinguish between an active or past infection, although in a recent study antibody response to H. pylori proteins such as VacA, GroEl, HcpC, CagA, Tip-α, HP1564 and HP0175 indicates an active H. pylori infection with a high diagnostic accuracy [81][82][81,82].
Detection of serum IgG against H. pylori is usually based on the enzyme-linked immunosorbent assays (ELISA). The latex immunoassay may be employed with the advantage to save time [83]. Several kits are available in the market and, overall, they are highly sensitive and specific; however, to maintain a high diagnostic accuracy, serologic tests need to be validated locally [84], especially when the kit uses strains’ antigens from different geographic areas [85]. Because IgG titers decline slowly (over around six months) the test is not recommended to evaluate bacterial eradication after treatment. Serology testing does not have the ability to distinguish active infections from past infections. In addition, the positive predictive value of antibody testing is affected by the local prevalence of H. pylori, especially in those areas where the H. pylori prevalence is inferior to 20%.
3.5.
Tests on plasma, blood, saliva and urine
The GastroPanel, especially the new-generation test, assesses simultaneously H. pylori antibodies and pepsinogen (PG) I plus PG II and gastrin-17 in the plasma, predicting H. pylori infection and the presence of atrophic gastritis with a likelihood of 94-95% [86]. The test is the most comprehensive non-invasive diagnostic test as it avoids false negative results respect to conventional tests [86][87][88][89][90][86-90]. A decreased PG1/2 ratio is associated with chronic atrophic gastritis and intestinal metaplasia (p < 0.001) and, inversely, an increased ratio correlates with gastritis [87]. The GastroPanel is useful under specific conditions. For example, in very old or fragile or with severe comorbidities patients, or healthy subjects from regions at low gastric cancer prevalence, but with gastric cancer familiarity who refuse to undergo upper endoscopy, evaluation with the GastroPanel may offer a comprehensive overview of the H. pylori and gastric mucosa status.
A plasma sample also offers the opportunity to detect circulating microRNAs (miRNAs) by molecular techniques. For example, the expression of four miR-28-3p, miR-143-3p, miR-151a-3p, and miR-148a-3p were found to be associated with H. pylori infection [91].
IgG antibodies against H. pylori may also be detected in dried blood spots, saliva and urine by ELISA with a reported good accuracy [92][93][94][92-94].
References
- Talley, N.J.; American Gastroenterological, A. American Gastroenterological Association medical position statement: evaluation of dyspepsia. Gastroenterology 2005, 129, 1753-1755, doi:10.1053/j.gastro.2005.09.019.
- Malfertheiner, P.; Megraud, F.; O'Morain, C.; Bazzoli, F.; El-Omar, E.; Graham, D.; Hunt, R.; Rokkas, T.; Vakil, N.; Kuipers, E.J. Current concepts in the management of Helicobacter pylori infection: the Maastricht III Consensus Report. Gut 2007, 56, 772-781, doi:10.1136/gut.2006.101634.
- Moayyedi, P.; Lacy, B.E.; Andrews, C.N.; Enns, R.A.; Howden, C.W.; Vakil, N. ACG and CAG Clinical Guideline: Management of Dyspepsia. Am J Gastroenterol 2017, 112, 988-1013, doi:10.1038/ajg.2017.154.
- El-Serag, H.B.; Kao, J.Y.; Kanwal, F.; Gilger, M.; LoVecchio, F.; Moss, S.F.; Crowe, S.E.; Elfant, A.; Haas, T.; Hapke, R.J.; et al. Houston Consensus Conference on Testing for Helicobacter pylori Infection in the United States. Clin Gastroenterol Hepatol 2018, 16, 992-1002 e1006, doi:10.1016/j.cgh.2018.03.013.
- Dore, M.P.; Pes, G.M.; Bassotti, G.; Usai-Satta, P. Dyspepsia: When and How to Test for Helicobacter pylori Infection. Gastroenterol Res Pract 2016, 2016, 8463614, doi:10.1155/2016/8463614.
- Luzza, F.; Pensabene, L.; Imeneo, M.; Mancuso, M.; Contaldo, A.; Giancotti, L.; La Vecchia, A.M.; Costa, M.C.; Strisciuglio, P.; Docimo, C.; et al. Antral nodularity identifies children infected with Helicobacter pylori with higher grades of gastric inflammation. Gastrointest Endosc 2001, 53, 60-64, doi:10.1067/mge.2001.111043.
- Bah, A.; Saraga, E.; Armstrong, D.; Vouillamoz, D.; Dorta, G.; Duroux, P.; Weber, B.; Froehlich, F.; Blum, A.L.; Schnegg, J.F. Endoscopic features of Helicobacter pylori-related gastritis. Endoscopy 1995, 27, 593-596, doi:10.1055/s-2007-1005764.
- Laine, L.; Cohen, H.; Sloane, R.; Marin-Sorensen, M.; Weinstein, W.M. Interobserver agreement and predictive value of endoscopic findings for H. pylori and gastritis in normal volunteers. Gastrointest Endosc 1995, 42, 420-423, doi:10.1016/s0016-5107(95)70043-9.
- Matrakool, L.; Tongtawee, T.; Bartpho, T.; Dechsukhum, C.; Loyd, R.A.; Kaewpitoon, S.J.; Kaewpitoon, N. Improved Detection of Helicobacter pylori Infection and Premalignant Gastric Mucosa Using Conventional White Light Source Gastroscopy. Asian Pac J Cancer Prev 2016, 17, 2099-2103, doi:10.7314/apjcp.2016.17.4.2099.
- Redeen, S.; Petersson, F.; Jonsson, K.A.; Borch, K. Relationship of gastroscopic features to histological findings in gastritis and Helicobacter pylori infection in a general population sample. Endoscopy 2003, 35, 946-950, doi:10.1055/s-2003-43479.
- Hatano, Y.; Haruma, K.; Kamada, T.; Shiotani, A.; Takahari, K.; Matsumoto, M.; Uchida, O. Factors Associated with Gastric Black Spot, White Flat Elevated Mucosa, and Cobblestone-Like Mucosa: A Cross-Sectional Study. Digestion 2018, 98, 185-193, doi:10.1159/000488796.
- Okamura, T.; Iwaya, Y.; Kitahara, K.; Suga, T.; Tanaka, E. Accuracy of Endoscopic Diagnosis for Mild Atrophic Gastritis Infected with Helicobacter pylori. Clin Endosc 2018, 51, 362-367, doi:10.5946/ce.2017.177.
- Tongtawee, T.; Kaewpitoon, S.; Kaewpitoon, N.; Dechsukhum, C.; Loyd, R.A.; Matrakool, L. Correlation between Gastric Mucosal Morphologic Patterns and Histopathological Severity of Helicobacter pylori Associated Gastritis Using Conventional Narrow Band Imaging Gastroscopy. Biomed Res Int 2015, 2015, 808505, doi:10.1155/2015/808505.
- Tahara, T.; Tahara, S.; Tuskamoto, T.; Horiguchi, N.; Yoshida, D.; Kawamura, T.; Okubo, M.; Nagasaka, M.; Nakagawa, Y.; Urano, M.; et al. Magnifying NBI Patterns of Gastric Mucosa After Helicobacter pylori Eradication and Its Potential Link to the Gastric Cancer Risk. Dig Dis Sci 2017, 62, 2421-2427, doi:10.1007/s10620-017-4676-x.
- Rugge, M.; Kim, J.G.; Mahachai, V.; Miehlke, S.; Pennelli, G.; Russo, V.M.; Perng, C.L.; Chang, F.Y.; Tandon, R.K.; Singal, D.K.; et al. OLGA gastritis staging in young adults and country-specific gastric cancer risk. Int J Surg Pathol 2008, 16, 150-154, doi:10.1177/1066896907307238.
- Saka, A.; Yagi, K.; Nimura, S. OLGA- and OLGIM-based staging of gastritis using narrow-band imaging magnifying endoscopy. Dig Endosc 2015, 27, 734-741, doi:10.1111/den.12483.
- Kotachi, T.; Ito, M.; Boda, T.; Kiso, M.; Masuda, K.; Hata, K.; Kawamura, T.; Sanomura, Y.; Yoshihara, M.; Tanaka, S.; et al. Clinical Significance of Reddish Depressed Lesions Observed in the Gastric Mucosa after Helicobacter pylori Eradication. Digestion 2018, 98, 48-55, doi:10.1159/000487045.
- Horiguchi, N.; Tahara, T.; Kawamura, T.; Okubo, M.; Tahara, S.; Nagasaka, M.; Nakagawa, Y.; Shibata, T.; Ohmiya, N. A Comparative Study of White Light Endoscopy, Chromoendoscopy and Magnifying Endoscopy with Narrow Band Imaging in the Diagnosis of Early Gastric Cancer after Helicobacter pylori Eradication. J Gastrointestin Liver Dis 2017, 26, 357-362, doi:10.15403/jgld.2014.1121.264.hpy.
- Horiguchi, N.; Tahara, T.; Yamada, H.; Yoshida, D.; Okubo, M.; Nagasaka, M.; Nakagawa, Y.; Shibata, T.; Tsukamoto, T.; Kuroda, M.; et al. In vivo diagnosis of early-stage gastric cancer found after Helicobacter pylori eradication using probe-based confocal laser endomicroscopy. Dig Endosc 2018, 30, 219-227, doi:10.1111/den.12926.
- Jiang, Z.X.; Nong, B.; Liang, L.X.; Yan, Y.D.; Zhang, G. Differential diagnosis of Helicobacter pylori-associated gastritis with the linked-color imaging score. Dig Liver Dis 2019, 51, 1665-1670, doi:10.1016/j.dld.2019.06.024.
- Nishikawa, Y.; Ikeda, Y.; Murakami, H.; Hori, S.I.; Hino, K.; Sasaki, C.; Nishikawa, M. Classification of atrophic mucosal patterns on Blue LASER Imaging for endoscopic diagnosis of Helicobacter pylori-related gastritis: A retrospective, observational study. PLoS One 2018, 13, e0193197, doi:10.1371/journal.pone.0193197.
- Ono, S.; Dohi, O.; Yagi, N.; Sanomura, Y.; Tanaka, S.; Naito, Y.; Sakamoto, N.; Kato, M. Accuracies of Endoscopic Diagnosis of Helicobacter pylori-Gastritis: Multicenter Prospective Study Using White Light Imaging and Linked Color Imaging. Digestion 2020, 101, 624-630, doi:10.1159/000501634.
- Osawa, H.; Miura, Y.; Takezawa, T.; Ino, Y.; Khurelbaatar, T.; Sagara, Y.; Lefor, A.K.; Yamamoto, H. Linked Color Imaging and Blue Laser Imaging for Upper Gastrointestinal Screening. Clin Endosc 2018, 51, 513-526, doi:10.5946/ce.2018.132.
- Wang, L.; Lin, X.C.; Li, H.L.; Yang, X.S.; Zhang, L.; Li, X.; Bai, P.; Wang, Y.; Fan, X.; Ding, Y.M. Clinical significance and influencing factors of linked color imaging technique in real-time diagnosis of active Helicobacter pylori infection. Chin Med J (Engl) 2019, 132, 2395-2401, doi:10.1097/CM9.0000000000000486.
- Zhu, Y.; Wang, F.; Zhou, Y.; Xia, G.L.; Dong, L.; He, W.H.; Xiao, B. Blue laser magnifying endoscopy in the diagnosis of chronic gastritis. Exp Ther Med 2019, 18, 1993-2000, doi:10.3892/etm.2019.7811.
- Sato, H.; Inoue, H.; Ikeda, H.; Sato, C.; Phlanusittepha, C.; Hayee, B.; Santi, E.G.; Kobayashi, Y.; Kudo, S.E. In vivo gastric mucosal histopathology using endocytoscopy. World J Gastroenterol 2015, 21, 5002-5008, doi:10.3748/wjg.v21.i16.5002.
- Toyoshima, O.; Nishizawa, T.; Koike, K. Endoscopic Kyoto classification of Helicobacter pylori infection and gastric cancer risk diagnosis. World J Gastroenterol 2020, 26, 466-477, doi:10.3748/wjg.v26.i5.466.
- Nakashima, H.; Kawahira, H.; Kawachi, H.; Sakaki, N. Artificial intelligence diagnosis of Helicobacter pylori infection using blue laser imaging-bright and linked color imaging: a single-center prospective study. Ann Gastroenterol 2018, 31, 462-468, doi:10.20524/aog.2018.0269.
- Bang, C.S.; Lee, J.J.; Baik, G.H. Artificial Intelligence for the Prediction of Helicobacter Pylori Infection in Endoscopic Images: Systematic Review and Meta-Analysis Of Diagnostic Test Accuracy. J Med Internet Res 2020, 22, e21983, doi:10.2196/21983.
- Dixon, M.F.; Genta, R.M.; Yardley, J.H.; Correa, P. Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am J Surg Pathol 1996, 20, 1161-1181, doi:10.1097/00000478-199610000-00001.
- Graham, D.Y.; Opekun, A.R.; Yamaoka, Y.; Osato, M.S.; el-Zimaity, H.M. Early events in proton pump inhibitor-associated exacerbation of corpus gastritis. Aliment Pharmacol Ther 2003, 17, 193-200, doi:10.1046/j.1365-2036.2003.01400.x.
- Glickman, J.N.; Noffsinger, A.; Nevin, D.T.; Ray, M.; Lash, R.H.; Genta, R.M. Helicobacter infections with rare bacteria or minimal gastritis: Expecting the unexpected. Dig Liver Dis 2015, 47, 549-555, doi:10.1016/j.dld.2015.04.005.
- Snead, D.R.; Tsang, Y.W.; Meskiri, A.; Kimani, P.K.; Crossman, R.; Rajpoot, N.M.; Blessing, E.; Chen, K.; Gopalakrishnan, K.; Matthews, P.; et al. Validation of digital pathology imaging for primary histopathological diagnosis. Histopathology 2016, 68, 1063-1072, doi:10.1111/his.12879.
- Godbole, G.; Megraud, F.; Bessede, E. Review: Diagnosis of Helicobacter pylori infection. Helicobacter 2020, 25 Suppl 1, e12735, doi:10.1111/hel.12735.
- Seo, J.H.; Park, J.S.; Rhee, K.H.; Youn, H.S. Limitations of urease test in diagnosis of pediatric Helicobacter pylori infection. World J Clin Pediatr 2015, 4, 143-147, doi:10.5409/wjcp.v4.i4.143.
- Cho, J.H.; Jeon, S.R.; Kim, H.G.; Jin, S.Y.; Park, S. Factors for improving the diagnostic efficiency of the rapid urease test from the gastric corpus. Scand J Gastroenterol 2017, 52, 1320-1325, doi:10.1080/00365521.2017.1378712.
- Parihar, V.; Holleran, G.; Hall, B.; Brennan, D.; Crotty, P.; McNamara, D. A combined antral and corpus rapid urease testing protocol can increase diagnostic accuracy despite a low prevalence of Helicobacter pylori infection in patients undergoing routine gastroscopy. United European Gastroenterol J 2015, 3, 432-436, doi:10.1177/2050640615573374.
- Kuhns, L.G.; Benoit, S.L.; Bayyareddy, K.; Johnson, D.; Orlando, R.; Evans, A.L.; Waldrop, G.L.; Maier, R.J. Carbon Fixation Driven by Molecular Hydrogen Results in Chemolithoautotrophically Enhanced Growth of Helicobacter pylori. J Bacteriol 2016, 198, 1423-1428, doi:10.1128/JB.00041-16.
- Peretz, A.; On, A.; Koifman, A.; Brodsky, D.; Isakovich, N.; Glyatman, T.; Paritsky, M. An efficiency comparison between three invasive methods for the diagnosis of Helicobacter pylori infections: Culture from stomach biopsy, rapid urease test (CUTest((R))), and histologic examination of gastric biopsy. Ann Clin Lab Sci 2015, 45, 148-151.
- Peretz, A.; Paritsky, M.; Pastukh, N.; Koifman, A.; Brodsky, D.; Glyatman, T.; On, A. Improvement and optimization of the classical gastric biopsy culture technique for Helicobacter pylori diagnosis using trypsin. J Med Microbiol 2015, 64, 642-645, doi:10.1099/jmm.0.000054.
- Pohl, D.; Keller, P.M.; Bordier, V.; Wagner, K. Review of current diagnostic methods and advances in Helicobacter pylori diagnostics in the era of next generation sequencing. World J Gastroenterol 2019, 25, 4629-4660, doi:10.3748/wjg.v25.i32.4629.
- Yin, S.M.; Zhang, F.; Shi, D.M.; Xiang, P.; Xiao, L.; Huang, Y.Q.; Zhang, G.S.; Bao, Z.J. Effect of posture on (13)C-urea breath test in partial gastrectomy patients. World J Gastroenterol 2015, 21, 12888-12895, doi:10.3748/wjg.v21.i45.12888.
- Klein, P.D.; Malaty, H.M.; Martin, R.F.; Graham, K.S.; Genta, R.M.; Graham, D.Y. Noninvasive detection of Helicobacter pylori infection in clinical practice: the 13C urea breath test. Am J Gastroenterol 1996, 91, 690-694.
- Agha, A.; Opekun, A.R.; Abudayyeh, S.; Graham, D.Y. Effect of different organic acids (citric, malic and ascorbic) on intragastric urease activity. Aliment Pharmacol Ther 2005, 21, 1145-1148, doi:10.1111/j.1365-2036.2005.02440.x.
- Eisdorfer, I.; Shalev, V.; Goren, S.; Chodick, G.; Muhsen, K. Sex differences in urea breath test results for the diagnosis of Helicobacter pylori infection: a large cross-sectional study. Biol Sex Differ 2018, 9, 1, doi:10.1186/s13293-017-0161-7.
- Kwon, Y.H.; Kim, N.; Lee, J.Y.; Choi, Y.J.; Yoon, K.; Hwang, J.J.; Lee, H.J.; Lee, A.; Jeong, Y.S.; Oh, S.; et al. The Diagnostic Validity of Citric Acid-Free, High Dose (13)C-Urea Breath Test After Helicobacter pylori Eradication in Korea. Helicobacter 2015, 20, 159-168, doi:10.1111/hel.12189.
- Suki, M.; Leibovici Weissman, Y.; Boltin, D.; Itskoviz, D.; Tsadok Perets, T.; Comaneshter, D.; Cohen, A.; Niv, Y.; Dotan, I.; Leibovitzh, H.; et al. Helicobacter pylori infection is positively associated with an increased BMI, irrespective of socioeconomic status and other confounders: a cohort study. Eur J Gastroenterol Hepatol 2018, 30, 143-148, doi:10.1097/MEG.0000000000001014.
- Graham, D.Y.; Miftahussurur, M. Helicobacter pylori urease for diagnosis of Helicobacter pylori infection: A mini review. J Adv Res 2018, 13, 51-57, doi:10.1016/j.jare.2018.01.006.
- Opekun, A.R.; Abdalla, N.; Sutton, F.M.; Hammoud, F.; Kuo, G.M.; Torres, E.; Steinbauer, J.; Graham, D.Y. Urea breath testing and analysis in the primary care office. J Fam Pract 2002, 51, 1030-1032.
- Richter, V.; Gonzalez, J.O.; Hazan, S.; Gottlieb, G.; Friedenberg, K.; Gatof, D.; Ganeshappa, R.; Delgado, J.S.; Abramowitz, D.; Hardi, R.; et al. The validity of breath collection bags method in detecting Helicobacter pylori using the novel BreathID ((R)) Hp Lab System: a multicenter clinical study in 257 subjects. Ther Adv Gastrointest Endosc 2019, 12, 2631774519843401, doi:10.1177/2631774519843401.
- Dore, M.P.; Osato, M.S.; Malaty, H.M.; Graham, D.Y. Characterization of a culture method to recover Helicobacter pylori from the feces of infected patients. Helicobacter 2000, 5, 165-168, doi:10.1046/j.1523-5378.2000.00026.x.
- Malfertheiner, P.; Megraud, F.; O'Morain, C.A.; Gisbert, J.P.; Kuipers, E.J.; Axon, A.T.; Bazzoli, F.; Gasbarrini, A.; Atherton, J.; Graham, D.Y.; et al. Management of Helicobacter pylori infection-the Maastricht V/Florence Consensus Report. Gut 2017, 66, 6-30, doi:10.1136/gutjnl-2016-312288.
- Ramirez-Lazaro, M.J.; Lite, J.; Lario, S.; Perez-Jove, P.; Montserrat, A.; Quilez, M.E.; Martinez-Bauer, E.; Calvet, X. Good diagnostic accuracy of a chemiluminescent immunoassay in stool samples for diagnosis of Helicobacter pylori infection in patients with dyspepsia. J Investig Med 2016, 64, 388-391, doi:10.1136/jim-2015-000004.
- Korkmaz, H.; Findik, D.; Ugurluoglu, C.; Terzi, Y. Reliability of stool antigen tests: investigation of the diagnostic value of a new immunochromatographic Helicobacter pylori approach in dyspeptic patients. Asian Pac J Cancer Prev 2015, 16, 657-660, doi:10.7314/apjcp.2015.16.2.657.
- Lario, S.; Ramirez-Lazaro, M.J.; Montserrat, A.; Quilez, M.E.; Junquera, F.; Martinez-Bauer, E.; Sanfeliu, I.; Brullet, E.; Campo, R.; Segura, F.; et al. Diagnostic accuracy of three monoclonal stool tests in a large series of untreated Helicobacter pylori infected patients. Clin Biochem 2016, 49, 682-687, doi:10.1016/j.clinbiochem.2016.01.015.
- Moubri, M.; Burucoa, C.; Kalach, N.; Larras, R.R.; Nouar, N.; Mouffok, F.; Arrada, Z. Performances of the IDEIA HpStAR Stool Antigen Test in Detection of Helicobacter pylori Infection Before and After Eradication Treatment in Algerian Children. J Trop Pediatr 2019, 65, 210-216, doi:10.1093/tropej/fmy035.
- Kakiuchi, T.; Okuda, M.; Hashiguchi, K.; Imamura, I.; Nakayama, A.; Matsuo, M. Evaluation of a Novel Stool Antigen Rapid Test Kit for Detection of Helicobacter pylori Infection. J Clin Microbiol 2019, 57, doi:10.1128/JCM.01825-18.
- Fang, Y.J.; Chen, M.J.; Chen, C.C.; Lee, J.Y.; Yang, T.H.; Yu, C.C.; Chiu, M.C.; Kuo, C.C.; Weng, Y.J.; Bair, M.J.; et al. Accuracy of rapid Helicobacter pylori antigen tests for the surveillance of the updated prevalence of H. pylori in Taiwan. J Formos Med Assoc 2020, 119, 1626-1633, doi:10.1016/j.jfma.2019.12.003.
- Dore, M.P.; Negrini, R.; Tadeu, V.; Marras, L.; Maragkoudakis, E.; Nieddu, S.; Simula, L.; Cherchi, G.B.; Massarelli, G.; Realdi, G. Novel monoclonal antibody-based Helicobacter pylori stool antigen test. Helicobacter 2004, 9, 228-232, doi:10.1111/j.1083-4389.2004.00228.x.
- Gisbert, J.P.; de la Morena, F.; Abraira, V. Accuracy of monoclonal stool antigen test for the diagnosis of H. pylori infection: a systematic review and meta-analysis. Am J Gastroenterol 2006, 101, 1921-1930, doi:10.1111/j.1572-0241.2006.00668.x.
- Guarner, J.; Kalach, N.; Elitsur, Y.; Koletzko, S. Helicobacter pylori diagnostic tests in children: review of the literature from 1999 to 2009. Eur J Pediatr 2010, 169, 15-25, doi:10.1007/s00431-009-1033-x.
- Bénéjat, L.; Ducournau, A.; Lehours, P.; Megraud, F. Real-time PCR for Helicobacter pylori diagnosis. The best tools available. Helicobacter 2018, 23, e12512, doi:10.1111/hel.12512.
- Redondo, J.J.; Keller, P.M.; Zbinden, R.; Wagner, K. A novel RT-PCR for the detection of Helicobacter pylori and identification of clarithromycin resistance mediated by mutations in the 23S rRNA gene. Diagn Microbiol Infect Dis 2018, 90, 1-6, doi:10.1016/j.diagmicrobio.2017.09.014.
- Talarico, S.; Safaeian, M.; Gonzalez, P.; Hildesheim, A.; Herrero, R.; Porras, C.; Cortes, B.; Larson, A.; Fang, F.C.; Salama, N.R. Quantitative Detection and Genotyping of Helicobacter pylori from Stool using Droplet Digital PCR Reveals Variation in Bacterial Loads that Correlates with cagA Virulence Gene Carriage. Helicobacter 2016, 21, 325-333, doi:10.1111/hel.12289.
- Bazin, T.; Nchare Mfondi, A.; Julie, C.; Emile, J.F.; Raymond, J.; Lamarque, D. Contribution of genetic amplification by PCR for the diagnosis of Helicobacter pylori infection in patients receiving proton pump inhibitors. United European Gastroenterol J 2018, 6, 1267-1273, doi:10.1177/2050640618787055.
- Morilla, A.; Melon, S.; Alvarez-Arguelles, M.E.; Armesto, E.; Villar, H.; de Ona, M. Utility of normalized genome quantification of Helicobacter pylori in gastric mucosa using an in-house real-time polymerase chain reaction. PLoS One 2017, 12, e0178674, doi:10.1371/journal.pone.0178674.
- Kalach, N.; Gosset, P.; Dehecq, E.; Decoster, A.; Spyckerelle, C.; Papadopolos, S.; Dupont, C.; Raymond, J. Usefulness of Gastric Biopsy-Based Real-Time Polymerase Chain Reaction for the Diagnosis of Helicobacter pylori Infection in Children. J Pediatr Gastroenterol Nutr 2015, 61, 307-312, doi:10.1097/MPG.0000000000000787.
- Matsumoto, H.; Shiotani, A.; Nishibayashi, H.; Kamada, T.; Kimura, T.; Fujimura, Y.; Nakato, R.; Murao, T.; Fujita, M.; Haruma, K. Molecular Detection of H. pylori Using Adherent Gastric Mucous to Biopsy Forceps. Helicobacter 2016, 21, 548-553, doi:10.1111/hel.12310.
- Hsieh, M.S.; Liu, C.J.; Hsu, W.H.; Li, C.J.; Tsai, P.Y.; Hu, H.M.; Shih, H.Y.; Lu, C.Y.; Yu, F.J.; Kuo, F.C.; et al. Gastric juice-based PCR assay: An alternative testing method to aid in the management of previously treated Helicobacter pylori infection. Helicobacter 2019, 24, e12568, doi:10.1111/hel.12568.
- Peng, X.; Song, Z.; He, L.; Lin, S.; Gong, Y.; Sun, L.; Zhao, F.; Gu, Y.; You, Y.; Zhou, L.; et al. Gastric Juice-Based Real-Time PCR for Tailored Helicobacter Pylori Treatment: A Practical Approach. Int J Med Sci 2017, 14, 595-601, doi:10.7150/ijms.18996.
- Piroozmand, A.; Soltani, B.; Razavizadeh, M.; Matini, A.H.; Moosavi, G.A.; Salehi, M.; Soltani, S. Comparison of gastric juice soluble triggering receptor expressed on myeloid cells and C-reactive protein for detection of Helicobacter pylori infection. Electron Physician 2017, 9, 6111-6119, doi:10.19082/6111.
- Talarico, S.; Korson, A.S.; Leverich, C.K.; Park, S.; Jalikis, F.G.; Upton, M.P.; Broussard, E.; Salama, N.R. High prevalence of Helicobacter pylori clarithromycin resistance mutations among Seattle patients measured by droplet digital PCR. Helicobacter 2018, 23, e12472, doi:10.1111/hel.12472.
- Nezami, B.G.; Jani, M.; Alouani, D.; Rhoads, D.D.; Sadri, N. Helicobacter pylori Mutations Detected by Next-Generation Sequencing in Formalin-Fixed, Paraffin-Embedded Gastric Biopsy Specimens Are Associated with Treatment Failure. J Clin Microbiol 2019, 57, doi:10.1128/JCM.01834-18.
- Zhou, L.; Zhao, F.; Hu, B.; Fang, Y.; Miao, Y.; Huang, Y.; Ji, D.; Zhang, J.; Xu, L.; Zhang, Y.; et al. A Creative Helicobacter pylori Diagnosis Scheme Based on Multiple Genetic Analysis System: Qualification and Quantitation. Helicobacter 2015, 20, 343-352, doi:10.1111/hel.12206.
- Trespalacios, A.A.; Rimbara, E.; Otero, W.; Reddy, R.; Graham, D.Y. Improved allele-specific PCR assays for detection of clarithromycin and fluoroquinolone resistant of Helicobacter pylori in gastric biopsies: identification of N87I mutation in GyrA. Diagn Microbiol Infect Dis 2015, 81, 251-255, doi:10.1016/j.diagmicrobio.2014.12.003.
- Yari, F.; Abiri, R.; Aryan, E.; Ahmadi Jouybari, T.; Navabi, J.; Alvandi, A. Loop-Mediated Isothermal Amplification as a Fast Noninvasive Method of Helicobacter pylori Diagnosis. J Clin Lab Anal 2016, 30, 464-470, doi:10.1002/jcla.21880.
- Beckman, E.; Saracino, I.; Fiorini, G.; Clark, C.; Slepnev, V.; Patel, D.; Gomez, C.; Ponaka, R.; Elagin, V.; Vaira, D. A Novel Stool PCR Test for Helicobacter pylori May Predict Clarithromycin Resistance and Eradication of Infection at a High Rate. J Clin Microbiol 2017, 55, 2400-2405, doi:10.1128/JCM.00506-17.
- Iannone, A.; Giorgio, F.; Russo, F.; Riezzo, G.; Girardi, B.; Pricci, M.; Palmer, S.C.; Barone, M.; Principi, M.; Strippoli, G.F.; et al. New fecal test for non-invasive Helicobacter pylori detection: A diagnostic accuracy study. World J Gastroenterol 2018, 24, 3021-3029, doi:10.3748/wjg.v24.i27.3021.
- Clines, N.; Beckman, E. Development of a high throughput human stool specimen processing method for a molecular Helicobacter pylori clarithromycin resistance assay. PLoS One 2019, 14, e0224356, doi:10.1371/journal.pone.0224356.
- Pichon, M.; Pichard, B.; Barrioz, T.; Plouzeau, C.; Croquet, V.; Fotsing, G.; Cheron, A.; Vuillemin, E.; Wangermez, M.; Haineaux, P.A.; et al. Diagnostic Accuracy of a Noninvasive Test for Detection of Helicobacter pylori and Resistance to Clarithromycin in Stool by the Amplidiag H. pylori+ClariR Real-Time PCR Assay. J Clin Microbiol 2020, 58, doi:10.1128/JCM.01787-19.
- Butt, J.; Blot, W.J.; Shrubsole, M.J.; Varga, M.G.; Hendrix, L.H.; Crankshaw, S.; Waterboer, T.; Pawlita, M.; Epplein, M. Performance of multiplex serology in discriminating active vs past Helicobacter pylori infection in a primarily African American population in the southeastern United States. Helicobacter 2020, 25, e12671, doi:10.1111/hel.12671.
- Shafaie, E.; Saberi, S.; Esmaeili, M.; Karimi, Z.; Najafi, S.; Tashakoripoor, M.; Abdirad, A.; Hosseini, M.E.; Mohagheghi, M.A.; Khalaj, V.; et al. Multiplex serology of Helicobacter pylori antigens in detection of current infection and atrophic gastritis - A simple and cost-efficient method. Microb Pathog 2018, 119, 137-144, doi:10.1016/j.micpath.2018.04.018.
- Kawai, S.; Arai, K.; Lin, Y.; Nishiyama, T.; Sasakabe, T.; Wang, C.; Miwa, H.; Kikuchi, S. Comparison of the detection of Helicobacter pylori infection by commercially available serological testing kits and the (13)C-urea breath test. J Infect Chemother 2019, 25, 769-773, doi:10.1016/j.jiac.2019.03.026.
- Miftahussurur, M.; Yamaoka, Y. Diagnostic Methods of Helicobacter pylori Infection for Epidemiological Studies: Critical Importance of Indirect Test Validation. Biomed Res Int 2016, 2016, 4819423, doi:10.1155/2016/4819423.
- Miwa, H.; Kikuchi, S.; Ohtaka, K.; Kobayashi, O.; Ogihara, A.; Hojo, M.; Nagahara, A.; Sato, N. Insufficient diagnostic accuracy of imported serological kits for Helicobacter pylori infection in Japanese population. Diagn Microbiol Infect Dis 2000, 36, 95-99, doi:10.1016/s0732-8893(99)00143-1.
- M, M.; D, S.; Paloheimo, L.; Hendolin, P.; Suovaniemi, O.; Syrjänen, K. Helicobacter pylori (Hp) IgG ELISA of the New-Generation GastroPanel(R) Is Highly Accurate in Diagnosis of Hp-Infection in Gastroscopy Referral Patients. Anticancer Res 2020, 40, 6387-6398, doi:10.21873/anticanres.14660.
- Lee, S.P.; Lee, S.Y.; Kim, J.H.; Sung, I.K.; Park, H.S.; Shim, C.S. Link between Serum Pepsinogen Concentrations and Upper Gastrointestinal Endoscopic Findings. J Korean Med Sci 2017, 32, 796-802, doi:10.3346/jkms.2017.32.5.796.
- Paloheimo, L.; Tiusanen, T.; Suovaniemi, O.; SyrjAnen, K. Serological Biomarker Test (GastroPanel((R))) in the Diagnosis of Functional Gastric Disorders, Helicobacter pylori and Atrophic Gastritis in Patients Examined for Dyspeptic Symptoms. Anticancer Res 2021, 41, 811-819, doi:10.21873/anticanres.14833.
- Syrjänen, K.; Eskelinen, M.; Peetsalu, A.; Sillakivi, T.; Sipponen, P.; Harkonen, M.; Paloheimo, L.; Maki, M.; Tiusanen, T.; Suovaniemi, O.; et al. GastroPanel(R) Biomarker Assay: The Most Comprehensive Test for Helicobacter pylori Infection and Its Clinical Sequelae. A Critical Review. Anticancer Res 2019, 39, 1091-1104, doi:10.21873/anticanres.13218.
- Tepes, B.; Seruga, M.; Vujasinovic, M.; Urlep, D.; Ljepovic, L.; Brglez, J.N.; Forte, A.; Anita Kek, L.; Skvarc, M. Premalignant Gastric Lesions in Patients Included in National Colorectal Cancer Screening. Radiol Oncol 2018, 52, 7-13, doi:10.1515/raon-2017-0054.
- Yu, J.; Xu, Q.; Zhang, X.; Zhu, M. Circulating microRNA signatures serve as potential diagnostic biomarkers for Helicobacter pylori infection. J Cell Biochem 2018, doi:10.1002/jcb.27462.
- Kumar, A.; Mhatre, S.; Dikshit, R. Utility of dried blood spots in detecting helicobacter pylori infection. Indian J Med Microbiol 2019, 37, 514-520, doi:10.4103/ijmm.IJMM_19_441.
- Okuda, M.; Mabe, K.; Lin, Y.; Chaochen, W.; Taniguchi, Y.; Kato, M.; Kikuchi, S. Rapid urine antibody test for Helicobacter pylori infection in adolescents. Pediatr Int 2017, 59, 798-802, doi:10.1111/ped.13286.
- Piroozmand, A.; Soltani, B.; Razavizadeh, M.; Matini, A.H.; Gilasi, H.R.; Zavareh, A.N.; Soltani, S. Comparison of the serum and salivary antibodies to detect gastric Helicobacter pylori infection in Kashan (Iran). Electron Physician 2017, 9, 6129-6134, doi:10.19082/6129.