1. Introduction
Due to the growing prevalence of diabetes worldwide, an increased incidence of premature deaths attributable to both diabetes as well as its complications is consequently to be expected
[1]. In 2017, approximately five million deaths in both developed and developing countries were reported which can directly be related to diabetes
[1]. The most common cardiovascular (CV) manifestations in individuals with type 2 diabetes mellitus (T2DM) included heart failure (HF), peripheral arterial disease, and coronary heart disease
[2]. Prevalence of HF in the patient population with established T2DM is twofold higher than in those without the disease
[3][4][3,4]. The Reykjavík Study has shown an overall prevalence of T2DM and HF of 0.5% in men and 0.4% in women
[5]. Therefore, the odds ratio (OR) for the association between T2DM and HF is 2.8 (95% CI = 2.2–3.6) and between abnormal glucose regulation and HF it is 1.7 (95% CI = 1.4–2.1)
[5].
Fatal and non-fatal CV outcomes, a risk of urgent hospitalization, and both short-term and long-term prognoses are sufficiently worse for T2DM patients when compared with those without T2DM
[6]. T2DM development coincides with numerous structural and functional changes in the heart, vessels, skeletal muscles, adipose tissue, kidney, and other target organs, which in the presence of traditional CV risk factors contribute to increased HF risk
[7][8][7,8]. Numerous clinical trials have revealed the synergistic effect of managing both HF and T2DM on their prognosis and clinical course
[9][10][11][9,10,11]. In this context, biomarkers that reflect various pathophysiological stages of T2DM progression might have promising potential in guiding therapies. In addition, biomarkers offer important diagnostic and predictive information, which cannot be derived from clinical observation or objective data evaluation
[12][13][12,13].
2. Basic Underlying Mechanisms of HF Development in Diabetics
Cardiac dysfunction in T2DM is a result of the development of metabolic abnormalities, attributed to increase fasting glucose, insulin resistance, lipotoxicity, and impaired reparation, sometimes termed diabetic cardiomyopathy, although not widely used
[14]. Other causes for the occurrence of HF in diabetics include conventional CV risk factors that include hypertension, dyslipidemia, abdominal obesity, asymptomatic atherosclerosis, CVD, chronic kidney disease (CKD), as well as non-traditional risk factors, such as ectopic calcification and osteoporosis
[15][16][17][15,16,17]. Consequently, cardiac dysfunction in T2DM patients is characterized by primary metabolic disturbances, secondary ischemic injury, cardiac myocyte apoptosis, immunological alterations with subsequent subcellular component abnormalities (mitochondrial stress, endoplasmic reticular formation dysfunction, secretome shaping impairment), oxidative stress with reduced nitric oxide bioavailability, fibrosis, local myocardial and microvascular inflammation, impaired cellular signaling (altered calcium homeostasis, activation of the sympathetic nervous system and the renin–angiotensin–aldosterone system (RAAS)), endothelial dysfunction, and altered tissue reparation
[16][17][16,17].
In fact, impaired glucose metabolism, lipotoxicity, altered metabolic memory, and insulin resistance are considered as key factors contributing to mitochondrial stress and myocardial cell injury
[17][18][17,18]. Indeed, suppressed AMP kinase activity due to mitochondrial dysfunction and consequently lowered phosphorylation of troponin relates to diastolic dysfunction prior to systolic dysfunction beyond the turnover of myosin chain isoforms
[19]. In addition, impaired diabetic cardiac function is a result of insulin-dependent activation of phosphatidylinositol 3-kinase (PI3K)/protein kinase B-ACT signaling, which alters titin phosphorylation and consequently leads to titin N2B/N2BA isoform modification and titin-based myocardial stiffness
[20][21][20,21]. There is a large body of evidence regarding the fact that insulin can directly stimulate the expression of a number of hypertrophic genes in cardiac myocyte including β-myosin heavy chain, insulin-like growth factor 1 receptor, myocyte enhancement factor, and brain-type natriuretic peptide (BNP)
[22][23][24][22,23,24]. Moreover, insulin-like growth factor 1, acting directly as an activator of the insulin receptor and indirectly through extracellular signal-regulated kinase 2 (Erk1/2) and PI3K signaling pathways, mediates cardiac hypertrophy, extracellular remodeling, and suppresses cardiac myocyte apoptosis
[25][26][25,26].
The development of cardiac dysfunction in T2DM is closely associated with hyperactivity of both the RAAS and sympatico-adrenal nervous system (SNS)
[27]. Acting as triggers of gluconeogenesis, lipolysis, and glycolysis, catecholamines, angiotensin-II and aldosterone promote the production of advanced glycation end products (AGE), which directly and along with insulin and glucose activate transforming growth factor beta 1 (TGF-β1)/SMAD signaling pathways through appropriate cell surface receptors (RAGE)
[28]. Consequently, increased oxidative stress, inflammatory response, and fibrotic extracellular matrix transformation with collagen cross-linking correspond to adverse cardiac remodeling, acceleration of atherosclerosis, and worsening vascular integrity and endothelial function
[29][30][31][29,30,31]. Of note, impaired GLUT4 and PI3K/Akt/eNOS signaling due to the activation of RAAS and insulin resistance accompany the reduced tyrosine phosphorylation of insulin receptor substrate (IRS)-1/2, which in turn leads to a lowered nitric oxide production. It directly impairs the vasomotor ability of coronary arteries, and substantially decreases in the recruitment, proliferation, and survival of endothelial progenitor cells that play a pivotal role in endogenous vascular reparation
[32].
Metabolic stress-induced pro-inflammatory activation has been cited as a powerful factor contributing to the pathogenesis of T2DM cardiomyopathy and HF
[33]. Numerous inflammatory cytokines, such as tumor necrosis factor-alpha (TNF-α), interleukin-6, as well as some adipocytokines, act as triggers for insulin resistance via the enhancement of nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) and fork-head box-containing protein O subfamily (FoxO1) expression, as well as c-Jun N terminal kinase (JNK) activation, that induce the phosphorylation of IRS-1 and hamper the activation of peroxisome proliferator-activated receptors (PPAR)-γ receptors
[34][35][34,35]. These perturbations mediate insulin resistance of the myocardium and skeletal muscles, adipose tissue inflammation, markedly reduce an interaction of FoxO1 with the promoter region of the β-isoform of myosin heavy chain (β-MHC) as well as negatively regulate β-MHC gene expression
[36].
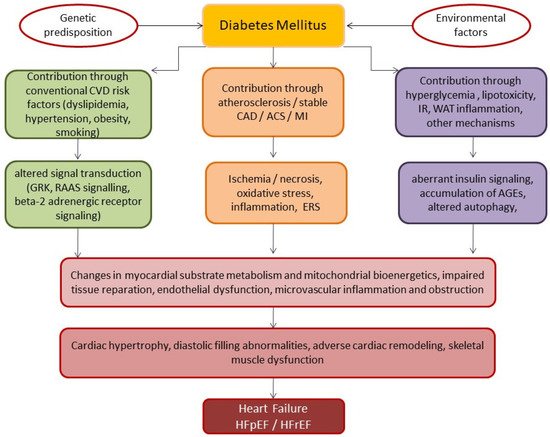
The structure and functional abnormalities result in adverse cardiac remodeling such as diastolic and systolic dysfunction due to cardiac hypertrophy, extracellular matrix accumulation and interstitial fibrosis, resulting in progressive dilated cardiomyopathy and decreased cardiac output, eventually leading to HF
[37]. The underlying pathophysiological mechanisms contributing to the development of HF in DM are reported in .
Figure 1. Underlying pathophysiological mechanisms contributing to the development of HF in patients with DM. Abbreviations: ACS, acute coronary syndrome; AGEs, advanced glycated end-products; CAD, coronary artery disease; CVD, cardiovascular disease; GRK, g-protein receptor kinase; ERS, endoplasmic reticulum stress; MI, myocardial infarction; HF, heart failure; HFrEF, heart failure with reduced ejection fraction; HFpEF, heart failure with preserved ejection fraction; IR, insulin resistance; RAAS, renin–angiotensin–aldosterone system; WAT, white adipose tissue.
Of note, there is evidence that approximately one-third of patients with T2DM demonstrate isolated diastolic filling abnormality and subclinical myocardial dysfunction, unrelated to accelerating atherosclerosis or CVD
[38]. Therefore, left ventricular (LV) diastolic dysfunction and LV concentric hypertrophy may be seen as the first signs of cardiac complications in patients with T2DM, independently from metabolic control
[39][40][39,40]. In addition, uncontrolled T2DM with hyperglycemic status, hyperinsulinemia/insulin resistance, and lipotoxicity may result in cardiotoxicity, acute and chronic LV systolic dysfunction in the absence of CAD, valvular, congenital, or hypertensive heart disease, or alcoholism
[41]. In fact, several phenotypes of T2DM-induced cardiac dysfunction may mostly relate to an overlap, but different alterations, which may be observed in failing hearts of T2DM patients. Indeed, substantial abnormalities in contractile (myosin, actin), regulatory (troponin, tropomyosin, and tropomodulin) and structural (predominantly titin, myomesin, and Ρ-actinin) protein expression are responsible for the occurrence of diastolic and systolic dysfunctions as a primary myocardial alteration. For example, a decreased Ca
2+ sensitivity along with a turnover of cardiac myosin heavy chain from V1 to V3 isoforms contributes to HFrEF
[42]. Thus, an imbalance between adaptive and maladaptive molecular mechanisms of cardiac metabolism and reparation secures a link between T2DM and cardiac dysfunction
[43][44][45][43,44,45]. MicroRNA and exosome-shaped transfer of active molecules are therefore also engaged in the pathogenesis of T2DM-induced cardiac dysfunction
[46].
3. Biomarkers in Diabetics with Known HF
Biomarkers have been posed as promising surrogate indictors of pathologic changes in target organs (myocardium, kidney, vessels, and skeletal muscles) and metabolic homeostasis, particularly having diagnostic and predictive capabilities for patients with T2DM and HF
[47]. Current clinical guidelines of the American College of Cardiology (ACC)/American Heart Association (AHA)/Heart Failure Society of America (HFSA) and European Cardiology Society (ESC) have proposed the use of biomarkers in personalized medical care of HF patients, regardless of T2DM, to diagnose HF and stratify patients at higher risk of poor prognosis, despite some differences in recommendations for practical use
[6][48][49][6,48,49]. reports the use of biomarkers in the management of HF according to 2016 ESC and 2017 ACC/AHA clinical guidelines
[6][48][6,48].
Table 1. 2016 ESC and 2017 ACC/AHA/HFSA recommendations for the use of biomarkers in the management of HF.
|
Strategy
|
Biomarkers
|
ESC, 2016
|
ACC/AHA/HFSA, 2017
|
|
COR
|
LOE
|
Phenotype of HF
|
COR
|
LOE
|
Phenotype of HF
|
|
Diagnosis
|
BNP/NT-proBNP/MR-proANP *
|
I
|
A
|
AHF, HFpEF, HFmrEF
|
I
|
A
|
AHF, CHF
|
|
Risk of in-hospital death
|
BNP/NT-proBNP
|
I
|
C
|
AHF
|
I
|
A
|
AHF, CHF
|
|
hs-cTr
|
I
|
C
|
AHF
|
I
|
A
|
AHF, CHF
|
|
Risk of recurrent hospital admission
|
BNP/NT-proBNP
|
-
|
I
|
A
|
AHF, CHF
|
|
Risk of post-discharged death
|
BNP/NT-proBNP
|
I
|
A
|
AHF, CHF
|
I
|
A
|
AHF, CHF
|
|
hs-cTr
|
I
|
C |
|
|
IIa |
|
|
| B |
|
| AHF, CHF
|
|
Guided therapy
|
BNP/NT-proBNP
|
-
|
I
|
A
|
HFrEF/HFpEF
|
Abbreviations: ACC, American College of Cardiology; AHA, American Heart Association; BNP, B-type natriuretic peptide; HF, heart failure; HFSA, Heart Failure Society of America; NT-proBNP, N-terminal pro-B-type natriuretic peptide; sST2, soluble ST2; COR, class of recommendation; LOE, level of evidence; MR-proANP, mid-regional pro A-type natriuretic peptide; hs-cTn, high-sensitivity cardiac troponins; HFrEF, heart failure reduced ejection fraction; HFpEF, heart failure preserved ejection fraction; HFmrEF, heart failure mid-range ejection fraction; *, provided for 2016 ESC recommendation only.
Although biomarkers of biomechanical stress (natriuretic peptides) and myocardial injury (high-sensitivity cardiac troponins (hs-cTn)), which are commonly used in HFrEF and to help to diagnose HFpEF, have high predictive utility in T2DM, they are markers of general pathological processes and consequently are not specific for T2DM-induced HF
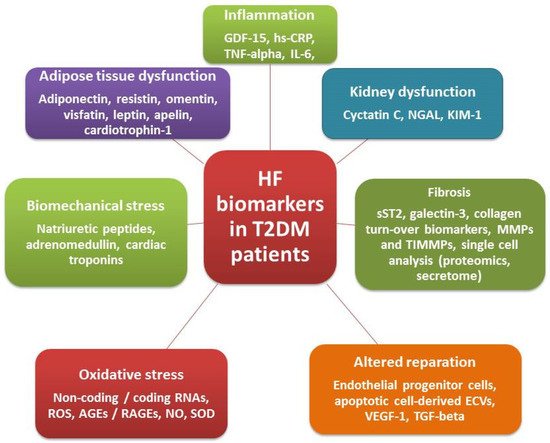
[50]. Conventional and alternative biomarkers of HF in T2DM patients are reported in .
Figure 2. Conventional and alternative biomarkers of HF in T2DM patients. Abbreviations: hs-CRP, high-sensitivity C-reactive protein; IL, interleukin; GDF, growth differential factor; NO, nitric oxide; SOD, superoxide dismutase; RNA, ribonucleic acid; ECVs, extracellular vesicles; VEGF, vascular endothelial growth factor; MMP, matrix metalloproteinase; TNF, tumor necrosis factor; TIMMP, tissue inhibitor of MMP; TGF, transforming growth factor; ROC, reactive oxygen species; AGEs, advanced glycation end products, RAGEs, receptor for AGEs; NGAL, neutrophil gelatinase-associated lipocalin; KIM-1, kidney injury molecule-1.
Novel biomarkers of fibrosis and inflammation (soluble suppression of tumorigenicity-2 (sST2) and galectin-3 (Gal-3)) are included in the ACC/AHA/HFSA HF guidelines as an alternative tool for CVD prediction and HF risk stratification, but their clinical utility in T2DM has not yet been proven and requires thorough elucidation. However, they were surrogate biomarkers for hard endpoints, such as all-cause and CV mortality and hospitalization in several large clinical trials in T2DM
[51]. Other alternative HF biomarkers, such as oxidative stress, inflammation, and collagen turn-over biomarkers, have been investigated in the context of offering add-on information for prognoses and personalized risk management among patients with T2DM-induced HF
[52][53][54][55][52,53,54,55]. reports the advantages and disadvantages of HF biomarkers in patients with T2DM.
Table 2. Advantages and disadvantages of HF biomarkers in T2DM patients.
|
Biomarkers
|
Underlying Pathophysiological Mechanisms
|
Possible Application for HF Phenotype
|
Advantages
|
Disadvantages
|
|
NPs
|
Biomechanical stress
|
HFrEF, HFpEF
|
Available for diagnosis, risk stratification, prognosis, and point-to-care therapy
|
High serum level variability, variable cut-off points in patients with AF, CKD, AO, prediction in HFrEF is higher than HFpEF
|
|
hs-cTn
|
Myocardial injury
|
Manly HFrEF
|
Available for risk stratification and prognosis
|
No add-on prediction to NPs
|
|
Mid-regional-pro-adrenomedullin
|
Neurohumoral activation
|
HFrEF, HFpEF
|
Better than NPs in predicting short-term mortality in acute HF
|
No superiority to NPs in predictive ability among chronic HFrEF/HFpEF
|
|
hs-CRP, IL-6
|
Inflammation
|
HFrEF, HFpEF
|
Prediction of all-cause mortality, CVD, HF-related events
|
Not suitable for point-of-care therapy, no ability to increase predictive ability of NPs, not recommended by reputed medical societies
|
|
GDF-15
|
Inflammation
|
HFrEF, HFpEF
|
Available for improving predictive ability of NPs, suitable for multiple biomarker strategy and point-of-care therapy
|
High cost, not recommended by reputed medical societies
|
|
|
|
sST2, galectin-3
|
Fibrosis/inflammation |
| AHF, CHF
|
I
|
IIa
|
HFpEF
|
Better than NPs for predicting mortality and HF-related events in non-HF patients, low individual serum level variability
AHF, CHF
|
|
| High cost |
|
Galectin-3
|
-
|
|
|
Collagen turn-over biomarkers
|
Fibrosis | IIb
|
B
|
AHF, CHF
|
|
|
| HFpEF
|
Available for risk stratification and prognosis
|
High cost, not recommended by reputed medical societies
|
sST2
|
-
|
IIb
|
B
|
AHF, CHF
|
|
Prevention of HF onset
|
BNP/NT-proBNP
|
-
|
Abbreviations: AO, abdominal obesity; AF, atrial fibrillation; CKD, chronic kidney disease; NPs, natriuretic peptides; hs-CRP, high-sensitivity C-reactive protein; sST2, soluble suppressor tumorigenisity-2; GDF-15, growth differential factor-15; IL, interleukin; HFrEF, heart failure with reduced ejection fraction; HFpEF, heart failure with preserved ejection fraction.
4. Multiple Biomarker Strategies
Multiple biomarker predictive models are considered as an effective method to increase the specificity and sensitivity of a single biomarker tool
[56][155]. Data confirm the superiority of multiple models compared with conventional models in risk stratification in HFpEF, whereas the adoption of serial biomarker measurements for risk stratification in HFpEF remains uncertain. However, different combinations of circulating cardiac biomarkers are likely a promising tool to improve prediction, risk stratification, and therapy in T2DM with HF, although there are limited data on the optimal number of biomarkers that can be allocated to improve point-of-care therapy among both HFrEF and HFpEF patients
[57][156]. There is no strong evidence that single biomarker use is superior to a multiple biomarker strategy for every clinical condition in HF patients. For instance, the MOLITOR (Impact of Therapy Optimisation on the Level of Biomarkers in Patients with Acute and Decompensated Chronic Heart Failure) study has shown that serial measurements of multiple biomarkers (C-terminal fragment of pre-pro-vasopressin, NT-proBNP, mid-regional pro-atrial natriuretic peptide, mid-regional pro-adrenomedullin, and C-terminal pro-endothelin-1) in advanced HF were no better than measurements of the C-terminal fragment of pre-pro-vasopressin
[58][157]. Pandey A. et al. (2021)
[59][158] evaluated the application of a biomarker-based risk score to identify patients with dysglycemia that were at high risk of incident HF. They enrolled individuals from three cohort studies (ARIC (Atherosclerosis Risk In Communities), DHS (Dallas Heart Study), and MESA (Multi-Ethnic Study of Atherosclerosis)). The original biomarker score included hs-cTnT ≥ 6 ng/L, NT-proBNP ≥ 125 pg/mL, hs-CRP ≥ 3 mg/L, and left ventricular hypertrophy identified by electrocardiography with one point for each abnormal parameter. The authors found that the 5-year risk for HF was associated with an increase in biomarker score; moreover, the highest risk was noted in patients with total scores of ≥3 (diabetes: 12.0%; pre-diabetes: 7.8%). Thus, it has been established that the biomarker score can stratify the HF risk among patients with T2DM and pre-diabetes. Berezin AE et al. (2019)
[60][159] reported that the combination of NT-proBNP and ST2 had higher prognostic ability when compared with each biomarker alone in patients with acute HF, except for galectin-3 and hs-CRP, which did not increase in discriminative potency when compared to a multiple biomarker model in ischemia-induced HF. Consequently, these conflicting results deserve closer investigation in large clinical trials in the future.
5. Point-of-Care Clinical Diagnostics in HF
In the clinical setting, the detection of individual biomarkers or a combined analysis thereof are promising tools to support the manifestation and diagnosis of cardiac diseases, such as HF. The ACC/Aha/HFSA guidelines (2017)
[6] have also recommended the measurement of additional biomarkers, such as sST-2 and galectin-3, for the risk assessment in HF. Although multiple assays are available to detect a vast number of biomarkers, they are most often rather expensive and time-consuming. For instance, as far as most enzyme-linked immunosorbent assays (ELISA) are concerned, several hours or one entire day are necessary to obtain the assay results, depending on the kit used. However, in point-of-care clinical diagnostics, it is crucial to obtain reliable results within a short time range. Multiple companies have addressed this challenge and have already successfully rolled out some rapid tests for biomarker analysis.