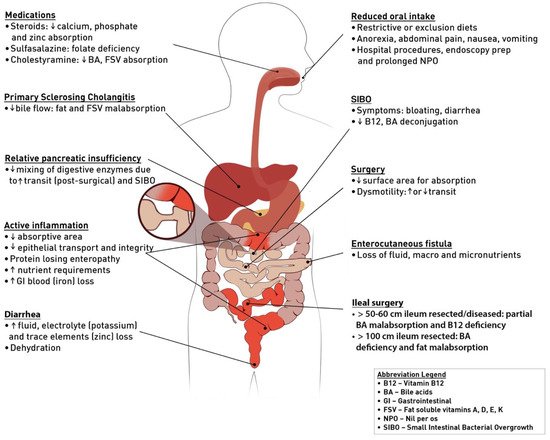
Malnutrition is highly prevalent in inflammatory bowel disease (IBD) patients and disproportionately affects those admitted to hospital. Malnutrition is a risk factor for many complications in IBD, including prolonged hospitalization, infection, greater need for surgery, development of venous thromboembolism, post-operative complications, and mortality. Early screening for malnutrition and prompt nutrition intervention if indicated has been shown to prevent or mitigate many of these outlined risk factors. There are many causes of malnutrition in IBD including reduced oral food intake, medications, active inflammation, and prior surgical resections. Hospitalization can further compound pre-existing malnutrition through inappropriate diet restrictions, nil per os (NPO) for endoscopy and imaging, or partial bowel obstruction, resulting in “post-hospital syndrome” after discharge and readmission.
- inflammatory bowel disease
- malnutrition
- nutrition support
- enteral nutrition
- peripheral parenteral nutrition
- central parenteral nutrition
- sarcopenia
1. Introduction
2. What Are the Clinical Implications of Malnutrition in Hospitalized IBD Patients?
3. How Can Clinicians Diagnose Malnutrition in IBD?
4. What Tools Can We Use to Screen All IBD Patients for Malnutrition?
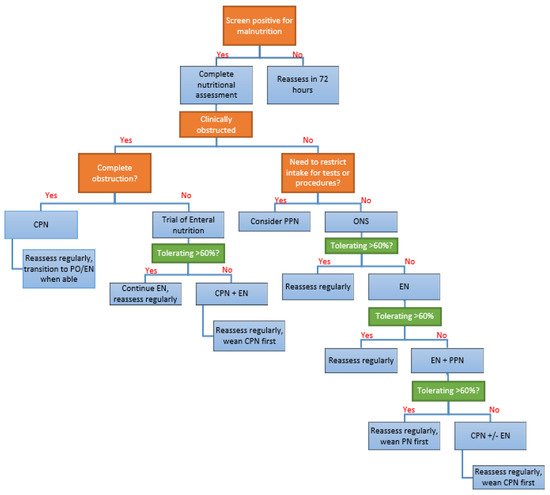
5. What Is the Approach to Nutrition Support in Hospitalized Patients with IBD?
6. How Should Nutrition Therapy Be Optimized in IBD Perioperatively?
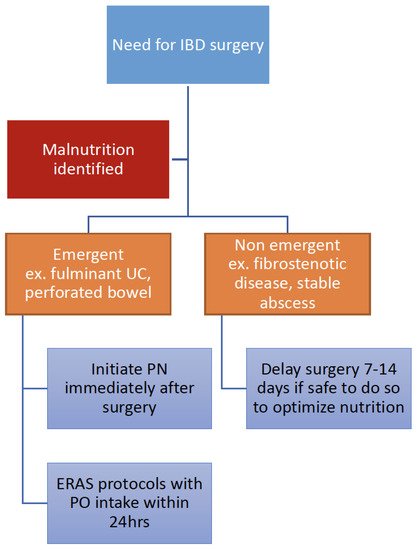
7. What Is the Role of Multidisciplinary Nutrition Care during Admission and After Discharge?
8. Conclusions and Future Directions
Table 1. Take-home clinical points.
|
Key points |
|
· Malnutrition is highly prevalent in IBD patients. |
|
· All hospitalized IBD patients should be screened for malnutrition. · Iatrogenic factors contributing to malnutrition in hospitals should be minimized. · Malnourished IBD patients should be treated with ONS, EN, PPN, CPN or some combination of these nutrition interventions. |
- (A)
- No gold standard definition of malnutrition in IBD and lack of validated NST and NAT
-
This applies to both hospitalized and community IBD populations. There is a wide range in the reported prevalence of malnutrition in IBD due to a lack of well-validated tools and wide variability in disease location and severity. A set of disease-specific yet encompassing criteria for malnutrition in IBD remains to be determined. Until further high-quality data are available, it is essential that all IBD patients admitted to hospitals be screened for malnutrition utilizing either the NRS-2002 or MUST NST with prompt nutritional interventions if at moderate to high risk of malnutrition.
- (B)
- Limited high-quality data for the use/timing of PN, including total versus supplemental, and central versus peripheral
-
While there are widely agreed-upon parameters for CPN use and timing, these are based on studies in non-IBD populations, using non-IBD specific nutritional risk assessment tools. Further studies of PN use, including timing and dosing, are warranted. There is a lack of data for when and how PPN can be optimized in patients with IBD. There are critical points early in hospital admission where patients may benefit from PPN as a bridge or supplement to longer-term therapy, but there are no data to guide this use. How IBD patients may benefit from PN requires further research.
- (C)
- Paucity of data on the impact of nutritional interventions on hospitalized IBD patients
-
While there is growing literature on the impact of nutrition interventions in improving IBD peri-operative risks, there remains a lack of high-quality studies for benefits of nutrition interventions on other outcomes of interest (infections, length of stay, readmission, surgery, etc.). Various nutritional therapies including ONS, EN, PPN, supplementary PN, and CPN were explored in this review, and recommendations for the sequenced use of nutritional therapies were provided based on data from other conditions.In summary, we outlined the high prevalence of malnutrition in IBD arising from its unique pathophysiology and the importance of malnutrition screening in all hospitalized IBD patients. We provide an expert opinion on diagnosing malnutrition in IBD that does not require weight loss or low BMI. Finally, we suggest approaches to nutrition therapies for the hospitalized IBD patient. Future prospective studies should prioritize these important areas of research to advance our understanding of how to best treat malnutrition in IBD.
References
- Seyedian, S.S.; Nokhostin, F.; Malamir, M.D. A review of the diagnosis, prevention, and treatment methods of inflammatory bowel disease. J. Med. Life 2019, 12, 113–122. [Google Scholar] [CrossRef]
- Ng, S.C.; Shi, H.Y.; Hamidi, N.; Underwood, F.E.; Tang, W.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Wu, J.C.Y.; Chan, F.K.L.; et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: A systematic review of population-based studies. Lancet 2017, 390, 2769–2778. [Google Scholar] [CrossRef]
- Piovani, D.; Danese, S.; Peyrin—Biroulet, L.; Bonovas, S. Inflammatory bowel disease: Estimates from the global burden of disease 2017 study. Aliment. Pharmacol. Ther. 2020, 51, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Frolkis, A.D.; Dykeman, J.; Negrón, M.E.; deBruyn, J.; Jette, N.; Fiest, K.M.; Frolkis, T.; Barkema, H.W.; Rioux, K.P.; Panaccione, R.; et al. Risk of Surgery for Inflammatory Bowel Diseases Has Decreased Over Time: A Systematic Review and Meta-analysis of Population-Based Studies. Gastroenterology 2013, 145, 996–1006. [Google Scholar] [CrossRef]
- Goh, J.; O’Morain, C.A. Nutrition and adult inflammatory bowel disease. Aliment. Pharmacol. Ther. 2003, 17, 307–320. [Google Scholar] [CrossRef] [PubMed]
- Donnellan, C.F.; Yann, L.H.; Lal, S. Nutritional management of Crohn’s disease. Therap. Adv. Gastroenterol. 2013, 6, 231–242. [Google Scholar] [CrossRef] [PubMed]
- Cederholm, T.; Barazzoni, R.; Austin, P.; Ballmer, P.; Biolo, G.; Bischoff, S.C.; Compher, C.; Correia, I.; Higashiguchi, T.; Holst, M.; et al. ESPEN guidelines on definitions and terminology of clinical nutrition. Clin. Nutr. 2016, 36, 49–64. [Google Scholar] [CrossRef]
- Corina, H.; Rami, E.; Raanan, S. Nutritional status and nutritional therapy in inflammatory bowel diseases. World J. Gastroenterol. 2009, 15, 2570–2578. [Google Scholar] [CrossRef]
- Han, P.D.; Burke, A.; Baldassano, R.N.; Rombeau, J.L.; Lichtenstein, G.R. Nutrition And Inflammatory Bowel Disease. Gastroenterol. Clin. N. Am. 1999, 28, 423–443. [Google Scholar] [CrossRef]
- Mijač, D.D.; Janković, G.L.J.; Jorga, J.; Krstić, M.N. Nutritional status in patients with active inflammatory bowel disease: Prevalence of malnutrition and methods for routine nutritional assessment. Eur. J. Intern. Med. 2010, 21, 315–319. [Google Scholar] [CrossRef]
- Nguyen, G.C.; Steinhart, A.H. Nationwide patterns of hospitalizations to centers with high volume of admissions for inflammatory bowel disease and their impact on mortality. Inflamm. Bowel. Dis. 2008, 14, 1688–1694. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyere, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef]
- Hollingworth, T.W.; Oke, S.M.; Patel, H.; Smith, T.R. Getting to grips with sarcopenia: Recent advances and practical management for the gastroenterologist. Frontline Gastroenterol. 2021, 12, 53–61. [Google Scholar] [CrossRef]
- Ryan, E.; McNicholas, D.; Creavin, B.; Kelly, M.E.; Walsh, T.; Beddy, D. Sarcopenia and Inflammatory Bowel Disease: A Systematic Review. Inflamm. Bowel Dis. 2019, 25, 67–73. [Google Scholar] [CrossRef]
- Scaldaferri, F.; Pizzoferrato, M.; Lopetuso, L.R.; Musca, T.; Ingravalle, F.; Sicignano, L.L.; Mentella, M.; Miggiano, G.; Mele, M.C.; Gaetani, E.; et al. Nutrition and IBD: Malnutrition and/or Sarcopenia? A Practical Guide. Gastroenterol. Res. Pract. 2017, 2017, 8646495. [Google Scholar] [CrossRef] [PubMed]
- Allard, J.P.; Keller, H.; Jeejeebhoy, K.N.; Laporte, M.; Duerksen, D.R.; Gramlich, L.; Payette, H.; Bernier, P.; Vesnaver, E.; Davidson, B.; et al. Malnutrition at Hospital Admission—Contributors and Effect on Length of Stay: A Prospective Cohort Study From the Canadian Malnutrition Task Force. JPEN J. Parenter Enteral. Nutr. 2016, 40, 487–497. [Google Scholar] [CrossRef]
- Kang, M.C.; Kim, J.H.; Ryu, S.W.; Moon, J.Y.; Park, J.H.; Park, J.K.; Park, J.H.; Baik, H.W.; Seo, J.M.; Son, M.W.; et al. Prevalence of Malnutrition in Hospitalized Patients: A Multicenter Cross-sectional Study. J. Korean Med. Sci. 2018, 33, e10. [Google Scholar] [CrossRef] [PubMed]
- Curtis, L.J.; Bernier, P.; Jeejeebhoy, K.; Allard, J.; Duerksen, D.; Gramlich, L.; Laporte, M.; Keller, H.H. Costs of hospital malnutrition. Clin. Nutr. 2016, 36, 1391–1396. [Google Scholar] [CrossRef] [PubMed]
- Tobert, C.M.M.D.; Mott, S.L.M.S.; Nepple, K.G.M.D.F. Malnutrition Diagnosis during Adult Inpatient Hospitalizations: Analysis of a Multi-Institutional Collaborative Database of Academic Medical Centers. J. Acad. Nutr. Diet. 2017, 118, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Felder, S.M.D.; Lechtenboehmer, C.; Bally, M.M.D.; Fehr, R.; Deiss, M.; Faessler, L.; Kutz, A.M.D.; Steiner, D.M.D.; Rast, A.C.M.D.; Laukemann, S.M.D.; et al. Association of nutritional risk and adverse medical outcomes across different medical inpatient populations. Nutrition 2015, 31, 1385–1393. [Google Scholar] [CrossRef]
- Lim, S.L.; Ong, K.C.B.; Chan, Y.H.; Loke, W.C.; Ferguson, M.; Daniels, L. Malnutrition and its impact on cost of hospitalization, length of stay, readmission and 3-year mortality. Clin. Nutr. 2011, 31, 345–350. [Google Scholar] [CrossRef]
- Rinninella, E.; Cintoni, M.; De Lorenzo, A.; Anselmi, G.; Gagliardi, L.; Addolorato, G.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. May nutritional status worsen during hospital stay? A sub-group analysis from a cross-sectional study. Intern. Emerg. Med. 2019, 14, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Ochoa Gautier, J.B. Quick Fix for Hospital-Acquired Malnutrition? JPEN J. Parenter Enteral. Nutr. 2016, 40, 302–304. [Google Scholar] [CrossRef]
- Casanova, M.J.; Chaparro, M.; Molina, B.; Merino, O.; Batanero, R.; Dueñas-Sadornil, C.; Robledo, P.; Garcia-Albert, A.M.; Gómez-Sánchez, M.B.; Calvet, X.; et al. Prevalence of Malnutrition and Nutritional Characteristics of Patients With Inflammatory Bowel Disease. J. Crohns Colitis 2017, 11, 1430–1439. [Google Scholar] [CrossRef]
- Ananthakrishnan, A.N.; McGinley, E.L.; Binion, D.G.; Saeian, K. A novel risk score to stratify severity of Crohn’s disease hospitalizations. Am. J. Gastroenterol. 2010, 105, 1799–1807. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, G.C.; Munsell, M.; Harris, M.L. Nationwide prevalence and prognostic significance of clinically diagnosable protein-calorie malnutrition in hospitalized inflammatory bowel disease patients. Inflamm. Bowel Dis. 2008, 14, 1105–1111. [Google Scholar] [CrossRef] [PubMed]
- Gajendran, M.; Umapathy, C.; Loganathan, P.; Hashash, J.G.; Koutroubakis, I.E.; Binion, D.G. Analysis of Hospital-Based Emergency Department Visits for Inflammatory Bowel Disease in the USA. Dig. Dis. Sci. 2016, 61, 389–399. [Google Scholar] [CrossRef]
- Wallaert, J.B.; De Martino, R.R.; Marsicovetere, P.S.; Goodney, P.P.; Finlayson, S.R.G.; Murray, J.J.; Holubar, S.D. Venous Thromboembolism After Surgery for Inflammatory Bowel Disease: Are There Modifiable Risk Factors? Data from ACS NSQIP. Dis. Colon Rectum. 2012, 55, 1138–1144. [Google Scholar] [CrossRef]
- Rahier, J.F.; Ben-Horin, S.; Chowers, Y.; Conlon, C.; de Munter, P.; D’Haens, G.; Domènech, E.; Eliakim, R.; Eser, A.; Frater, J.; et al. European evidence-based Consensus on the prevention, diagnosis and management of opportunistic infections in inflammatory bowel disease. J. Crohns Colitis 2009, 3, 47–91. [Google Scholar] [CrossRef] [PubMed]
- Philipson, T.; Snider, J.T.; Lakdawalla, D.N.; Stryckman, B.; Goldman, D.P. OP015 impact of oral nutritional supplementation on hospital outcomes. Clin. Nutr. 2013, 32, S6–S7. [Google Scholar] [CrossRef]
- Gomes, F.; Schuetz, P.; Bounoure, L.; Austin, P.; Ballesteros-Pomar, M.; Cederholm, T.; Fletcher, J.; Laviano, A.; Norman, K.; Poulia, K.-A.; et al. ESPEN guidelines on nutritional support for polymorbid internal medicine patients. Clin. Nutr. 2018, 37, 336–353. [Google Scholar] [CrossRef] [PubMed]
- Detsky, A.S.; McLaughlin, J.R.; Baker, J.P.; Johnston, N.; Whittaker, S.; Mendelson, R.A.; Jeejeebhoy, K.N. What is subjective global assessment of nutritional status? 1987. Classical article. Nutr. Hosp. 2008, 23, 400–407. [Google Scholar] [PubMed]
- Bian, D.; Shi, Y.; Jiang, Y.; Zhong, J.; Sun, J.; Gu, Y. Combined Patient-Generated Subjective Global Assessment and body composition facilitates nutritional support in inflammatory bowel disease: An ambulatory study in Shanghai. Asia Pac. J. Clin. Nutr. 2018, 27, 1230–1238. [Google Scholar] [CrossRef]
- Lu, Z.L.; Wang, T.R.; Qiao, Y.Q.; Zheng, Q.; Sun, Y.; Lu, J.T.; Han, X.X.; Fan, Z.P.; Ran, Z.H. Handgrip Strength Index Predicts Nutritional Status as a Complement to Body Mass Index in Crohn’s Disease. J. Crohns Colitis 2016, 10, 1395–1400. [Google Scholar] [CrossRef]
- Cederholm, T.; Bosaeus, I.; Barazzoni, R.; Bauer, J.; Van Gossum, A.; Klek, S.; Muscaritoli, M.; Nyulasi, I.; Ockenga, J.; Schneider, S.M.; et al. Diagnostic criteria for malnutrition—An ESPEN Consensus Statement. Clin. Nutr. 2015, 34, 335–340. [Google Scholar] [CrossRef]
- White, J.V.; Guenter, P.; Jensen, G.; Malone, A.; Schofield, M.; Academy Malnutrition Work Group; A.S.P.E.N. Malnutrition Task Force; A.S.P.E.N. Board of Directors. Consensus Statement of the Academy of Nutrition and Dietetics/American Society for Parenteral and Enteral Nutrition: Characteristics Recommended for the Identification and Documentation of Adult Malnutrition (Undernutrition). J. Acad. Nutr. Diet. 2012, 112, 730–738. [Google Scholar] [CrossRef] [PubMed]
- Kondrup, J.; Allison, S.P.; Elia, M.; Vellas, B.; Plauth, M. ESPEN Guidelines for Nutrition Screening 2002. Clin. Nutr. 2003, 22, 415–421. [Google Scholar] [CrossRef]
- Mueller, C.; Compher, C.; Ellen, D.M.; American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.) Board of Directors. A.S.P.E.N. clinical guidelines. J. Parenter. Enteral Nutr. 2011, 35, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Bischoff, S.C.; Escher, J.; Hébuterne, X.; Kłęk, S.; Krznaric, Z.; Schneider, S.; Shamir, R.; Stardelova, K.; Wierdsma, N.; Wiskin, A.E.; et al. ESPEN practical guideline: Clinical Nutrition in inflammatory bowel disease. Clin. Nutr. 2020, 39, 632–653. [Google Scholar] [CrossRef]
- Eide, H.D.; Halvorsen, K.; Almendingen, K. Barriers to nutritional care for the undernourished hospitalised elderly: Perspectives of nurses. J. Clin. Nurs. 2015, 24, 696–706. [Google Scholar] [CrossRef]
- Fjeldstad, S.H.; Thoresen, L.; Mowé, M.; Irtun, Ø. Changes in nutritional care after implementing national guidelines-a 10-year follow-up study. Eur. J. Clin. Nutr. 2018, 72, 1000–1006. [Google Scholar] [CrossRef]
- Ross, L.J.; Mudge, A.M.; Young, A.M.; Banks, M. Everyone’s problem but nobody’s job: Staff perceptions and explanations for poor nutritional intake in older medical patients: Hospital nutrition: Everyone’s problem, nobody’s job. Nutr. Diet. 2011, 68, 41–46. [Google Scholar] [CrossRef]
- Wanniarachige, D. Malnourished patients often unacknowledged. CMAJ 2015, 187, 242. [Google Scholar] [CrossRef]
- Suominen, M.H.; Sandelin, E.; Soini, H.; Pitkala, K.H. How well do nurses recognize malnutrition in elderly patients? Eur. J. Clin. Nutr. 2009, 63, 292–296. [Google Scholar] [CrossRef] [PubMed]
- Anthony, P.S. Nutrition Screening Tools for Hospitalized Patients. Nutr. Clin. Pract. 2008, 23, 373–382. [Google Scholar] [CrossRef]
- Schuetz, P.; Fehr, R.; Baechli, V.; Geiser, M.; Deiss, M.; Gomes, F.; Kutz, A.; Tribolet, P.; Bregenzer, T.; Braun, N.; et al. Individualised nutritional support in medical inpatients at nutritional risk: A randomised clinical trial. Lancet 2019, 393, 2312–2321. [Google Scholar] [CrossRef]
- Raslan, M.; Gonzalez, M.C.; Gonçalves Dias, M.C.; Nascimento, M.; Castro, M.; Marques, P.; Segatto, S.; Torrinhas, R.S.; Cecconello, I.; Waitzberg, D.L. Comparison of nutritional risk screening tools for predicting clinical outcomes in hospitalized patients. Nutrition 2010, 26, 721–726. [Google Scholar] [CrossRef]
- Stratton, R.J.; King, C.L.; Stroud, M.A.; Jackson, A.A.; Elia, M. ‘Malnutrition Universal Screening Tool’ predicts mortality and length of hospital stay in acutely ill elderly. Br. J. Nutr. 2007, 95, 325–330. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Ney, M.; Eslamparast, T.; Vandermeer, B.; Ismond, K.P.; Kroeker, K.; Halloran, B.; Raman, M.; Tandon, P. Systematic review of nutrition screening and assessment in inflammatory bowel disease. World J. Gastroenterol. 2019, 25, 3823–3837. [Google Scholar] [CrossRef]
- Takaoka, A.; Sasaki, M.; Nakanishi, N.; Kurihara, M.; Ohi, A.; Bamba, S.; Andoh, A. Nutritional Screening and Clinical Outcome in Hospitalized Patients with Crohn’s Disease. Ann. Nutr. Metab. 2017, 71, 266–272. [Google Scholar] [CrossRef]
- Bamba, S.; Sasaki, M.; Takaoka, A.; Takahashi, K.; Imaeda, H.; Nishida, A.; Inatomi, O.; Sugimoto, M.; Andoh, A. Sarcopenia is a predictive factor for intestinal resection in admitted patients with Crohn’s disease. PLoS ONE 2017, 12, e0180036. [Google Scholar] [CrossRef]
- Keetarut, K.; Zacharopoulou-Otapasidou, S.; Bloom, S.; Majumdar, A.; Patel, P.S. An evaluation of the feasibility and validity of a patient-administered malnutrition universal screening tool (MUST) compared to healthcare professional screening in an inflammatory bowel disease (IBD) outpatient clinic. J. Hum. Nutr. Diet. 2017, 30, 737–745. [Google Scholar] [CrossRef] [PubMed]
- Sandhu, A.; Mosli, M.; Yan, B.; Wu, T.; Gregor, J.; Chande, N.; Ponich, T.; Beaton, M.; Rahman, A. Self-Screening for Malnutrition Risk in Outpatient Inflammatory Bowel Disease Patients Using the Malnutrition Universal Screening Tool (MUST). JPEN J. Parenter. Enteral Nutr. 2016, 40, 507–510. [Google Scholar] [CrossRef]
- Haskey, N.; Pena-Sanchez, J.N.; Jones, J.L.; Fowler, S.A. Development of a screening tool to detect nutrition risk in patients with inflammatory bowel disease. Asia Pac. J. Clin. Nutr. 2018, 27, 756–762. [Google Scholar] [CrossRef] [PubMed]
- Jansen, I.; Prager, M.; Valentini, L.; Büning, C. Inflammation-driven malnutrition: A new screening tool predicts outcome in Crohn’s disease. Br. J. Nutr. 2016, 116, 1061–1067. [Google Scholar] [CrossRef]
- Sigall-Boneh, R.; Levine, A.; Lomer, M.; Wierdsma, N.; Allan, P.; Fiorino, G.; Gatti, S.; Jonkers, D.; Kierkus, J.; Katsanos, K.H.; et al. Research Gaps in Diet and Nutrition in Inflammatory Bowel Disease. A Topical Review by D-ECCO Working Group [Dietitians of ECCO]. J. Crohns Colitis 2017, 11, 1407–1419. [Google Scholar] [CrossRef] [PubMed]
- Platek, M.E.; Hertroijs, D.F.L.; Nicholson, J.M.; Parekh, N. Sensitivity and Specificity of Malnutrition Screening Tools Used in the Adult Hospitalized Patient Setting: A Systematic Review. Top. Clin. Nutr. 2015, 30, 289–301. [Google Scholar] [CrossRef]
- Tinsley, A.; Ehrlich, O.G.; Hwang, C.; Issokson, K.; Zapala, S.; Weaver, A.; Siegel, C.A.; Melmed, G.Y. Knowledge, Attitudes, and Beliefs Regarding the Role of Nutrition in IBD Among Patients and Providers. Inflamm. Bowel Dis. 2016, 22, 2474–2481. [Google Scholar] [CrossRef] [PubMed]
- Costa-Santos, M.P.P.C.; Torres, J.; Ferreira, A.; Velho, S.; Ourô, S.; Glória, L.; Gordo, I.; Maio, R.; Cravo, M. Preoperative enteral nutrition in adults with complicated Crohn’s disease: Effect on disease outcomes and gut microbiota. Nutr. X 2020, 5, 100009. [Google Scholar] [CrossRef]
- Forbes, A.; Escher, J.; Hébuterne, X.; Kłęk, S.; Krznaric, Z.; Schneider, S.; Shamir, R.; Stardelova, K.; Wierdsma, N.; Wiskin, A.E.; et al. ESPEN guideline: Clinical nutrition in inflammatory bowel disease. Clin. Nutr. 2017, 36, 321–347. [Google Scholar] [CrossRef]
- Yamamoto, T.; Nakahigashi, M.; Shimoyama, T.; Umegae, S. Does preoperative enteral nutrition reduce the incidence of surgical complications in patients with Crohn’s disease? A case—Matched study. Colorectal. Dis. 2020, 22, 554–561. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.-B.; Peng, X.; Xie, X.-Y.; Lian, L.; Wu, X.-R.; Hu, J.-C.; He, X.-W.; Ke, J.; Chen, Y.-F.; Zhi, M.; et al. Enteral nutrition is associated with a decreased risk of surgical intervention in Crohn’s disease patients with spontaneous intra-abdominal abscess. Rev. Esp. Enferm. Dig. 2017, 109, 834–842. [Google Scholar] [CrossRef]
- Day, A.; Wood, J.; Melton, S.; Bryant, R.V. Exclusive enteral nutrition: An optimal care pathway for use in adult patients with active Crohn’s disease. JGH Open 2020, 4, 260–266. [Google Scholar] [CrossRef]
- Worthington, P.; Balint, J.; Bechtold, M.; Bingham, A.; Chan, L.-N.; Durfee, S.; Jevenn, A.K.; Malone, A.; Mascarenhas, M.; Robinson, D.T.; et al. When Is Parenteral Nutrition Appropriate? JPEN J. Parenter. Enteral Nutr. 2017, 41, 324–377. [Google Scholar] [CrossRef]
- Gura, K.M. Is There Still a Role for Peripheral Parenteral Nutrition? Nutr. Clin. Pract. 2009, 24, 709–717. [Google Scholar] [CrossRef] [PubMed]
- Lappas, B.M.; Patel, D.; Kumpf, V.; Adams, D.W.; Seidner, D.L. Parenteral Nutrition: Indications, Access, and Complications. Gastroenterol. Clin. N. Am. 2018, 47, 39–59. [Google Scholar] [CrossRef]
- Comeche, J.M.; Comino, I.; Altavilla, C.; Tuells, J.; Gutierrez-Hervas, A.; Caballero, P. Parenteral Nutrition in Patients with Inflammatory Bowel Disease Systematic Review, Meta-Analysis and Meta-Regression. Nutrients 2019, 11, 2865. [Google Scholar] [CrossRef] [PubMed]
- Zangenberg, M.S.; Horesh, N.; Kopylov, U.; El-Hussuna, A. Preoperative optimization of patients with inflammatory bowel disease undergoing gastrointestinal surgery: A systematic review. Int. J. Colorectal. Dis. 2017, 32, 1663–1676. [Google Scholar] [CrossRef]
- Ge, X.; Tang, S.; Yang, X.; Liu, W.; Ye, L.; Yu, W.; Xu, H.; Cao, Q.; Zhou, W.; Cai, X. The role of exclusive enteral nutrition in the preoperative optimization of laparoscopic surgery for patients with Crohn’s disease: A cohort study. Int. J. Surg. 2019, 65, 39–44. [Google Scholar] [CrossRef]
- Ayoub, F.; Kamel, A.Y.; Ouni, A.; Chaudhry, N.; Ader, Y.; Tan, S.; Iqbal, A.; Zimmermann, E.M.; Glover, S.C. Pre-operative total parenteral nutrition improves post-operative outcomes in a subset of Crohn’s disease patients undergoing major abdominal surgery. Gastroenterol. Rep. 2019, 7, 107–114. [Google Scholar] [CrossRef]
- Schwartz, E. Perioperative Parenteral Nutrition in Adults With Inflammatory Bowel Disease: A Review of the Literature. Nutr. Clin. Pract. 2016, 31, 159–170. [Google Scholar] [CrossRef] [PubMed]
- Krumholz, H.M. Post-Hospital Syndrome—An Acquired, Transient Condition of Generalized Risk. N. Engl. J. Med. 2013, 368, 100–102. [Google Scholar] [CrossRef] [PubMed]
- Kaegi-Braun, N.; Mueller, M.; Schuetz, P.; Mueller, B.; Kutz, A. Evaluation of Nutritional Support and In-Hospital Mortality in Patients With Malnutrition. JAMA Netw. Open 2021, 4, e2033433. [Google Scholar] [CrossRef] [PubMed]
- Berkowitz, S.A.; Terranova, J.; Randall, L.; Cranston, K.; Waters, D.B.; Hsu, J. Association Between Receipt of a Medically Tailored Meal Program and Health Care Use. JAMA Intern. Med. 2019, 179, 786–793. [Google Scholar] [CrossRef]
- Marr, K.J.; Shaheen, A.-A.; Lam, L.; Stapleton, M.; Burak, K.; Raman, M. Nutritional status and the performance of multiple bedside tools for nutrition assessment among patients waiting for liver transplantation: A Canadian experience. Clin. Nutr. ESPEN 2016, 17, 68–74. [Google Scholar] [CrossRef]
