Magnesium-based hydrides are considered as promising candidates for solid-state hydrogen storage and thermal energy storage, due to their high hydrogen capacity, reversibility, and elemental abundance of Mg. To improve the sluggish kinetics of MgH2, catalytic doping using Ti-based catalysts is regarded as an effective approach to enhance Mg-based materials.
- magnesium hydride
- titanium-based hydride
- catalysis
- hydrogen storage properties
1. Introduction
2. Fundamentals of the Mg-H2 System
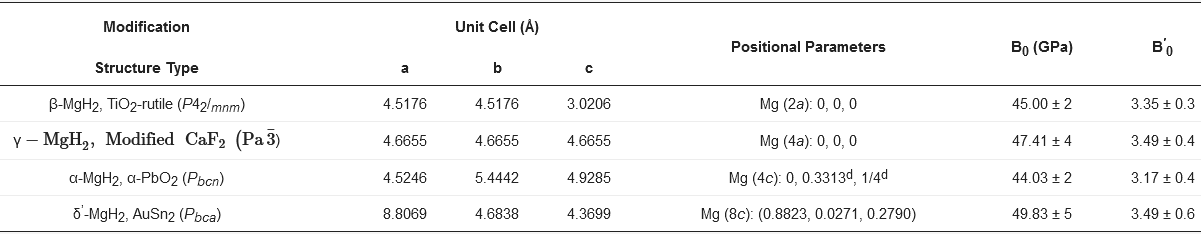
2.1. Crystal Structure
2.2. Thermodynamics of the Mg-H2 System
|
Thermodynamic Parameters |
Values |
|---|---|
|
Formation enthalpy, kJ/(mol·H2) |
|
Metal |
Dissociation Energy (eV) |
|---|---|
−74.5 | |
|
Pure Mg |
0.87, 0.40, 0.50, 1.15, 1.05, 0.95, 1.00 |
|
Ti-doped Mg |
Null, negligible |
|
Ni-doped Mg |
0.06 |
|
V-doped Mg |
Null |
|
Cu-doped Mg |
0.56 |
|
Pd-doped Mg |
0.39 |
|
Fe-doped Mg |
0.03 |
|
Ag-doped Mg |
1.18 |
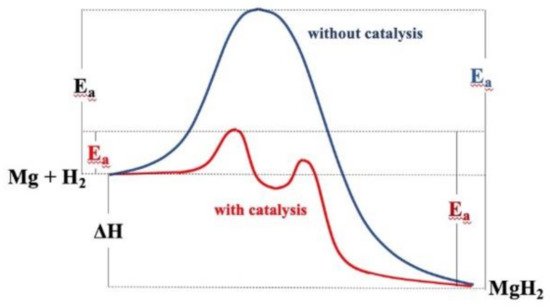
3. Catalytic Effects
3.1. Transition Metals Catalysts
|
Materials |
Synthetic Methods |
Hydrogen Storage Properties |
Reference |
|||||
|---|---|---|---|---|---|---|---|---|
|
Desorption Kinetics |
Eades (kJ/mol) |
Absorption Kinetics |
Eaabs (kJ/mol) |
|||||
|
Titanium/Titanium Hydrides |
||||||||
|
Mg-2%Ti |
Inert gas condensation |
Des: 4.50%/320 °C/0.2 bar/25 min |
Abs: 4.80%/320 °C/8 bar/21 min |
|||||
Formation entropy, J/(mol·H2·K) |
||||||||
|
MgH2 |
−135 |
|||||||
+ 2 at% Ti |
Ball milling (argon) |
Des: 6.32 wt%/623 K/35 kPa/0.5 h |
Abs: 6.32 wt%/623 K/2000 kPa/4 min |
Hydrogen Storage Capacity (Theoretical) |
||||
|
Gravimetric capacity, wt% | ||||||||
|
Cold rolling (5 times, air) |
Des: 6.00 wt%/623 K/35 kPa/0.5 h |
Abs: 5.70 wt%/623 K/2000 kPa/4 min |
7.6 |
|||||
|
MgH2-4 mol% Ti |
Ball milling |
Des: 1.10%/573 K/2 MPa/5 min |
Abs: 6.40%/573 K/2 MPa/5 min |
Volumetric capacity, g/(L·H2) |
110 |
|||
|
Thermal Energy Storage Capacity (Theoretical) |
||||||||
|
Gravimetric capacity, kJ/kg |
2204 |
|||||||
|
Volumetric capacity, kJ/dm3 |
1763 |
|||||||
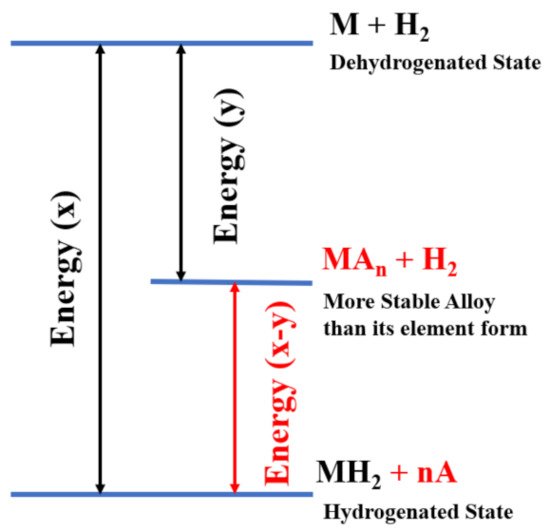
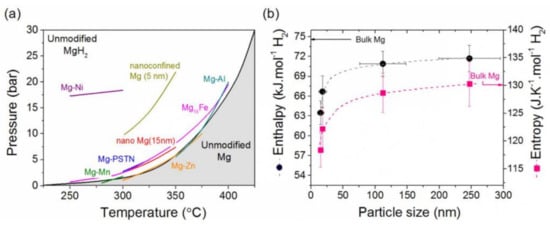
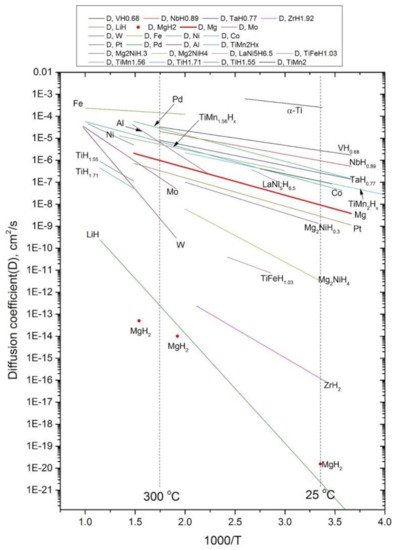
2.3. Kinetics
MgH | ||||||
2 | ||||||
-5 at% Ti |
Ball milling |
Des Temperature: 235.6 °C |
70.11 |
|||
|
MgH2-5 at% Ti |
Ball milling |
Des: 5.50%/523 K/0.015 MPa/20 min |
71.1 |
Abs: 4.20%/373 K/1.0 MPa/15 min |
||
|
MgH2-5 at% Ti |
Ball milling |
Des: 5.20%/573 K/0.03 MPa/15 min |
Abs: 6.70%/ 573 K/0.8 MPa/15 min |
|||
|
Mg-5% Ti |
Chemical vapor synthesis |
104 |
[59] |
|||
|
Mg-14 at% Ti |
Gas phase condensation |
35 |
52 |
[60] |
||
|
Mg-22 at% Ti |
31 |
47 |
||||
|
MgH2-15% Ti |
Ball milling |
Des: 0.12%/573 K/1 bar/60 min |
Abs: 3.48%/573 K/12 bar/60 min |
[61] |
||
|
Mg0.9Ti0.1 |
Ball milling |
76 |
Abs: 6.62% (after milling) |
[62] |
||
|
Mg0.75Ti0.25 |
Ball milling |
88 |
Abs: 6.18% (after milling) |
|||
|
Mg0.5Ti0.5 |
Ball milling |
91 |
Abs: 5.21% (after milling) |
|||
|
MgH2-20% Ti |
Ball milling |
72 ± 3 |
[63] |
|||
|
MgH2-coated Ti |
Ball milling |
Des: 5.00%/250 °C/15 min (TPD) Des Temperature: 175 °C |
[64] |
|||
|
Mg83.5Ti16.5 |
Inert gas condensation |
Des: 2.50%/300 °C/0.15 bar/2 min |
Abs: 2.20%/300 °C/9 bar/1 min |
[65] |
||
|
15Mg-Ti |
Chemical method |
72.2 |
[66] |
|||
|
MgH2-4 mol% TiH2 |
Ball milling |
Des: 0.70%/573 K/2 MPa/5 min |
Abs: 6.10%/573 K/2 MPa/5 min |
|||
|
MgH2-5 at% TiH2 |
Ball milling |
Des: 5.80%/270 °C/0.12 bar/10 min Des Temperature: 235.5 °C |
67.24 |
Abs: 2.70%/25 °C/1 bar/250 min |
||
|
10MgH2-TiH2 |
Ball milling |
73 |
[67] |
|||
|
7MgH2-TiH2 |
Ball milling |
71 |
[68] |
|||
|
4MgH2-TiH2 |
Ball milling |
68 |
[68] |
|||
|
MgH2-10 mol% TiH2 |
Ball milling |
Abs: 5.70%/240 °C/2 MPa/200 s |
16.4 |
[69] |
||
|
MgH2-10% TiH2 |
Ball milling |
24.2 |
[70] |
|||
|
MgH2-10% TiH2 |
Ball milling |
17.9 |
[71] |
|||
|
Mg-9.2% TiH1.971-3.7% TiH1.5 |
Ball milling |
Des: 4.10%/573 K/100 Pa/20 min |
46.2 |
Abs: 4.30%/298 K/4 MPa/10 min |
12.5 |
[72] |
|
Mg0.65Ti0.35D1.2 |
Ball milling |
17 |
[73] |
|||
|
Titanium Oxides |
||||||
|
MgH2-10% TiO2 |
Ball milling |
Des: 6.00%/300 °C/vacuum/20 min |
Abs: 6.00%/300 °C/0.84 MPa/5 min |
[74] |
||
|
Mg-20% TiO2 |
Reactive ball milling |
Des: 4.40%/350 °C/1 bar/8.5 min |
Abs: 3.80%/350 °C/20 bar/2 min |
[75] |
||
|
MgH2-6% TiO2 |
Ball milling |
145.8 ± 14.2 |
[76] |
|||
|
MgH2 + 10% TiO2 |
Ball milling |
Des Temperature: 200 °C |
75.50 |
[77] |
||
|
Titanium Halides |
||||||
|
MgH2-10% TiF4 |
Ball milling |
Des Temperature: 216.7 °C |
71 |
(Des: 6.6%) |
[78] |
|
|
MgH2-10% TiF4 |
Ball milling (2 h, argon) |
Des Temperature: 154 °C |
70 |
[79] |
||
|
MgH2 + 10% TiF4 |
Ball milling |
Des Temperature: 150 °C |
70 |
[77] |
||
|
MgH2-4 mol% TiF3 |
Ball milling |
Des: 4.50%/573 K/2 MPa/5 min |
Abs: 5.10%/573 K/2 MPa/5 min |
|||
|
MgH2-4 mol% TiCl3 |
Ball milling |
Des: 3.70%/573 K/2 MPa/5 min |
Abs: 5.30%/573 K/2 MPa/5 min |
|||
|
MgH2-7% TiCl3 |
Ball milling |
Des temperature: 274 °C |
85 |
[80] |
||
|
Titanium Alloys |
||||||
|
MgH2-5a% TiAl |
Ball milling |
Des: 4.90%/270 °C/0.12 bar/10 min Des Temperature: 219.6 °C |
65.08 |
Abs: 2.50%/25 °C/1 bar/250 min |
||
|
MgH2-5 a% Ti3Al |
Ball milling |
Des Temperature: 232.3 °C |
70.61 |
|||
|
Mg85Al7.5Ti7.5 |
DC-magnetron co-sputtering |
Des: 5.30%/200 °C/vacuum/20 min |
Abs: 5.60%/200 °C/3 bar/0.5 min |
[81] |
||
|
Mg0.63Ti0.27Si0.10D1.1 |
Ball milling |
27 |
[73] |
|||
|
MgH2-5 at%TiNi |
Ball milling |
Des Temperature: 242.4 °C |
73.09 |
|||
|
15Mg-Ti-0.75Ni |
Chemical method |
63.7 |
[66] |
|||
|
Mg0.63Ti0.27Ni0.10D1.3 |
Ball milling |
21 |
[73] |
|||
|
MgH2-5at%TiNb |
Ball milling |
Des: 5.90%/27 °C/0.12 bar/10 min Des Temperature: 231.3 °C |
71.72 |
Abs: 2.80%/25 °C/1 bar/250 min |
||
|
MgH2-5at% Cr-5a% Ti |
Film |
Des: 6.00%/200 °C/5 mbar/25 min |
Abs: 6.20%/200 °C/3 bar/10 min |
[82] |
||
|
MgH2-7 at% Cr-13 at% Ti |
Film |
Des: 5.00%/200 °C/5 mbar/25 min |
Abs: 5.60%/200 °C/3 bar/10 min |
|||
|
MgH2-5 at% TiFe |
Ball milling |
Des: 5.20%/270 °C/0.12 bar/10 min Des Temperature: 237.7 °C |
72.63 |
Abs: 3.00%/25 °C/1 bar/250 min |
||
|
MgH2-5% FeTi |
Ball milling |
Abs: 2.30%/150 °C/2 MPa/5 min |
21 |
[83] |
||
|
MgH2-5 at% TiMn2 |
Ball milling |
Des: 4.80%/270 °C/0.12 bar/10 min Des Temperature: 219.7 °C |
74.22 |
Abs: 3.20%/25 °C/1 bar/250 min |
||
|
MgH2-10% TiMn2 |
Ball milling |
22.6 |
[70] |
|||
|
MgH2-5% VTi |
Ball milling |
Abs: 3.30%/150 °C/2 MPa/5 min |
10.4 |
[83] |
||
|
Mg87.5Ti9.6V2.9 |
Hydrogen plasma metal reaction |
Des: 4.00%/300 °C/1 mbar/5 min |
73.8 |
Abs: 4.80%/200 °C/40 bar/5 min |
29.2 |
[84] |
|
MgH2-5 at% TiVMn |
Ball milling |
Des: 5.70%/270 °C/0.12 bar/10 min Des Temperature: 216.7 °C |
85.20 |
Abs: 3.00%/25 °C/1 bar/250 min |
||
|
Multiple Catalysts |
||||||
|
Mg-10% Ti-10% Pd |
Ball milling |
114 ± 4 |
[85] |
|||
|
Mg-TiH1.971-TiH1.5-ZrH1.66 |
Arc melting |
36.6 |
21.2 |
[86] |
||
|
Mg0.9Ti0.1 + 5% C |
Ball milling |
88 |
Abs: 6.43% (after milling) |
[62] |
||
|
MgH2-6% NiTiO3 |
Ball milling |
74 ± 4 |
[87] |
|||
|
MgH2-6% CoTiO3 |
Ball milling |
100 ± 2 |
||||
|
MgH2-10 mol% TiH2-6 mol% TiO2 |
Ball milling |
118 |
[88] |
|||
|
MgH2-5% VTi-CNTs |
Ball milling |
Abs: 5.10%/150 °C/2 MPa/5 min |
10.2 |
[83] |
||
|
MgH2-5% FeTi-CNTs |
Ball milling |
Abs: 0.60%/150 °C/2 MPa/5 min |
65.5 |
[83] |
||
|
MgH2-10% Ni-TiO2 |
Ball milling |
Des: 6.50%/265 °C/0.02 bar/7 min |
43.7 ± 1.5 |
Abs: 5.00%/100 °C/60 bar/7 min |
[76] |
|
|
MgH2-4% Ni-6% TiO2 |
Ball milling |
91.6 ± 8.5 |
[76] |
|||
|
MgH2-10% Co-TiO2 |
Ball milling |
Des: 6.20%/250 °C/0.02 bar/15 min |
77 |
Abs: 4.24%/100 °C/60 bar/10 min |
[89] |
|
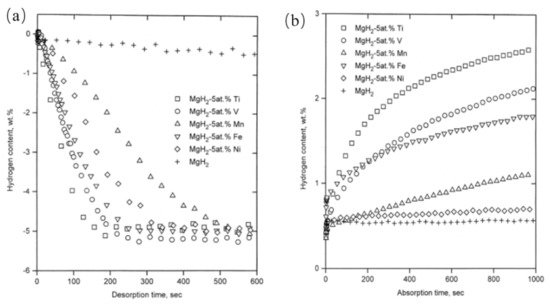
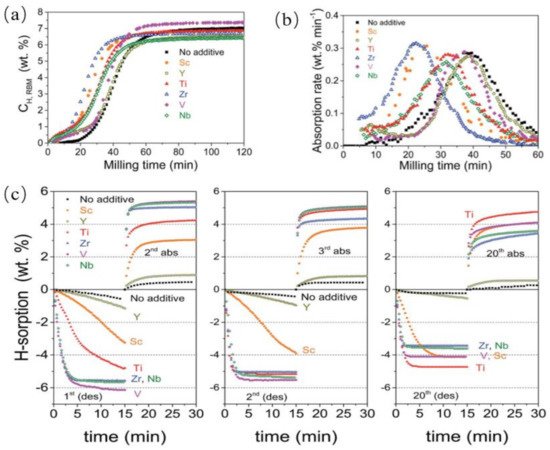
3.2. Catalytic Effects of Ti-Based Compounds
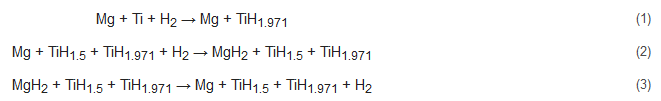

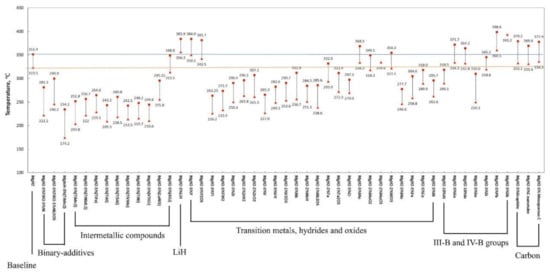
4. Synthetic Approaches
5. Mechanisms of Catalysis
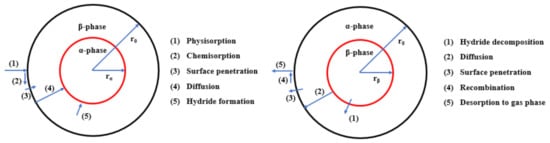
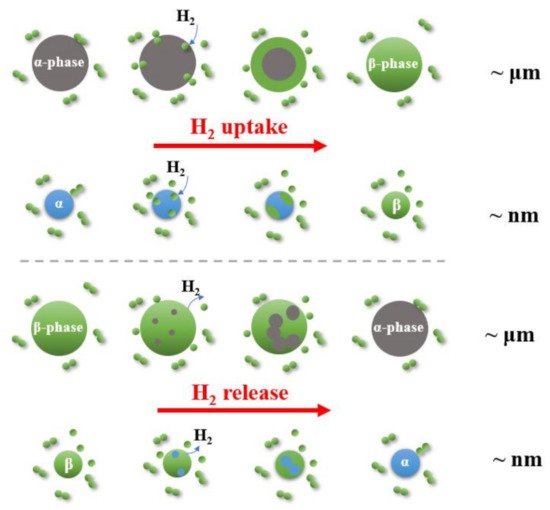
Understanding the catalysis is critical to improving hydrogen absorption and desorption kinetics for Mg-based systems. Based on the understanding of the hydrogen reaction in the metal-hydrogen system [113][140], the hydrogenation of metal should go through the following five steps: (1) Physisorption of the H2 molecule, (2) dissociation of the H2 molecule, (3) surface penetration of H atoms, (4) diffusion of H atoms in the host lattice, and (5) hydride formation at metal/hydride interface, as shown in Figure 9. For the dehydrogenation reaction, a hydride particle could go through the following steps: (1) Hydride decomposition, (2) diffusion of hydrogen atom, (3) surface penetration, (4) recombination to hydrogen molecule, and (5) desorption to the gas phase. Either hydrogen absorption or desorption should be controlled by a rate-limiting step while other steps are likely in equilibrium.