Antimicrobial resistance is mushrooming as a silent pandemic. It is considered among the most common priority areas identified by both national and international agencies. The global development of multidrug-resistant strains now threatens public health care improvement by introducing antibiotics against infectious agents.
- antimicrobial resistance
- ESKAPE
- bacteria
- antibiotics
1. Introduction
2. Drivers of Antimicrobial Resistance
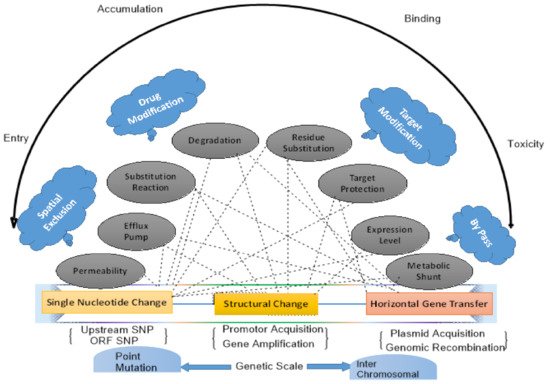
3. Global Dissemination of Antibiotic Resistance
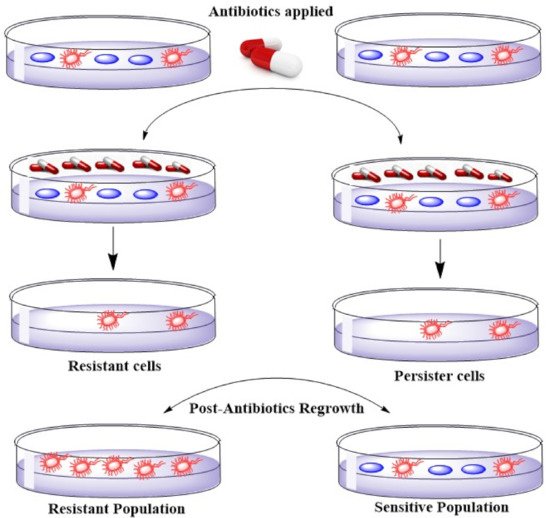
4. Emerging Resistance–Development of Resistant Strains
5. ESKAPE, Healthcare Concomitant Bugs–Bad Bugs with No Drugs
Bacterial Strain | Gram Staining Type | Resistance | Type | Antibiotics | Treatment Option | Resistance Level | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Acinetobacter | Negative | Multidrug | Ceftazidime, aminoglycoside, fluoroquinolones, carbapenems | Carbapenems, b-Lactamase inhibitors, Tigecycline, | Aminoglycosides , Polymyxin therapy, Synergy, and combination therapy | High level | |||||||||||||
Inactivation of Drug | Use of hydrolysis or modification | b-lactamase for b-lactam resistance, acetyltransferases for aminoglycoside resistance | |||||||||||||||||
E. coli | Negative | Multidrug | Cephalosporins (ESBL-producers), fluoroquinolones, aminoglycosides | GyrB/ParE programme, | EV-035 | High level | |||||||||||||
K. pneumoniae |
Nanoparticles (NP) | Mode of Action/Mechanism of Nanoparticles Against ESKAPE Pathogens | Antibiotic Used | Microorganism | Synergic Effects | (Antibiotics-Nanoparticles) | Ref. |
|---|
[ | ] |
AgNPs | Damage the bacterial cell membrane and disrupt the activity of membranous enzymes. Cell wall distraction by cell DNA was condensed to a tension state and could have lost its replicating abilities | [ |
Doxycycline | 54] | |||||||||||||||||||||||
K. pneumoniae | Observed |
[68] |
Alteration of Target | ||||||||||||||||||||||||
Gentamicin and Neomycin | Reduction of binding affinity to the drug by bypassing the drug target | DNA gyrase mutation for fluoroquinolone resistance | S. aureus | [ |
AgNPs + Gentamicin showed resistance in 50% strains while AgNPs + Neomycin showed synergy 45% of the strains. | 55] |
|||||||||||||||||||||
[ | ] |
Negative | Multidrug | Drug influx Reduction | By decreasing permeability | Gram-negative outer membrane | Cephalosporins (ESBL-producers), fluoroquinolones, aminoglycosides, carbapenems | ||||||||||||||||||||
E. coli, S. aureus | [ |
Observed increase in activity was such that Erythromycin showed 18.9.6%, Kanamycin = 27.9.3%, Chloramphenicol = 18.1.3%, and Ampicillin = 74.8.9% | 56] |
||||||||||||||||||||||||
[ | POL7080 and ACHN-975 compounds | High level | |||||||||||||||||||||||||
] | P. aeruginosa | Negative | Multidrug | Piperacillin/tazobactam, ceftazidime, ciprofloxacin, aminoglycosides, carbapenems | POL7080 and ACHN-975 compounds | High level | |||||||||||||||||||||
Extrusion of Drug | |||||||||||||||||||||||||||
β-Lactam, cefotaxime | Efflux pumps | E. coli, S. aureus | Accessory membrane fusion proteins |
[ |
Synergistic increase in activity was such that 17.2%, 13.5% for 57] | Enterococcus spp. | Positive | Multidrug | Ampicillin, aminoglycosides, glycopeptides | ||||||||||||||||||
Horizontal gene transfer | By resistance determinants from other microorganisms |
[58]RX-04 lead series, 50S ribosomal subunit; inhibit translation by stabilizing a distorted mode of P-tRNA binding | High level | ||||||||||||||||||||||||
S. aureus | Positive, | Multidrug | β-lactam antibiotics (except new anti- methicillin-resistant S. aureus cephalosporins), macrolides, fluoroquinolones, aminoglycosides | RX-04 lead series, 50S ribosomal subunit; inhibit translation by stabilizing a distorted mode of P-tRNA binding | High level |
6. General Mechanism of Antimicrobial Resistance
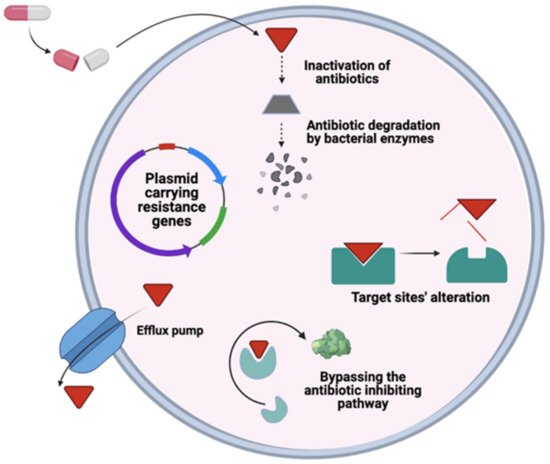
Resistance | Proposed Mechanism | Examples | Ref. |
|---|
7. Alternative Mechanisms for Combating Multidrug Resistance in ESKAPE Pathogens
7.1. CRISPR-Cas9
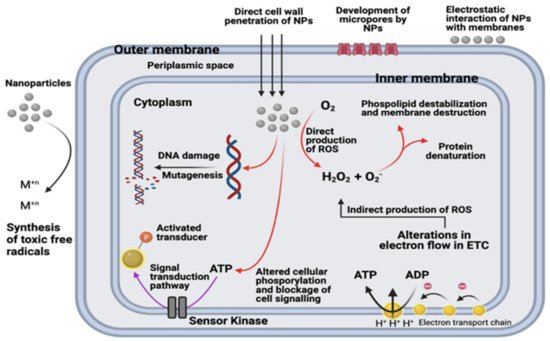
7.2. Nanotechnology and Nanoparticles to Combat Multidrug Resistance
E. coli | ||||||||||||||||
and | ||||||||||||||||
S. aureus | ||||||||||||||||
, respectively | ||||||||||||||||
[ | 70] |
|||||||||||||||
Ampicillin, chloramphenicol, and kanamycin | S. aureus, E. coli, | and P. aeruginosa | Synergistic effects observed |
[71] |
||||||||||||
Beta-lactam: cephem | S. aureus | Cephalothin and cefazolin showed a 30% increase in activity when used in combination with 20 μg/ mL AgNPs against Micrococcus luteus, and Bacillus subtilis |
[72] |
|||||||||||||
AuNPs | Disturb membrane potential by inhibiting ATPase activities; inhibit the subunit of the ribosome from binding tRNA. Cellular death induced by gold nanoparticles do not include reactive oxygen species-based mechanisms | Ampicillin, streptomycin, and kanamycin | E. coli and S. aureus | 15%, 12%, and 34% increase in inhibition zone for E. coli with A/S/K+Au, respectively; 20%, 109%, and 18% increase in inhibition zone for M. luteus A/S/K+AuNPs, respectively; 12% and 34% increase in inhibition zone for S. aureus with A/ K+AuNPs, respectively |
[73] |
|||||||||||
Beta lactams: cefaclor | S. aureus and E. coli | MICs of cefaclor reduced gold nanoparticles were 10 mg/mL and 100 mg/mL for S. aureus and E. coli, respectively |
[74] |
|||||||||||||
ZnONPs | Interactions between reactive oxygen species and membrane proteins result in cell damage. ZnO-NPs disrupt bacterial cell membrane integrity, reduce cell surface hydrophobicity, and down-regulate the transcription of oxidative stress-resistance genes in bacteria | Ceftriaxone | E. coli | Synergistic antibacterial effects against E. coli have been observed by ZnO nanorods with ceftriaxone |
[75] |
|||||||||||
Ciprofloxacin | S. aureus and E. coli | Increase in inhibition zones in S. aureus = 27% and 22% in E. coli when ciprofloxacin and ZnONPs were applied in synergism |
[76] |
|||||||||||||
Beta lactams, aminoglycosides, and azolides | S. aureus | The highest increase was observed for penicillin G and amikacin, i.e., 10 mm increase in the zone of inhibition, whereas for clarithromycin, a 2 mm increase had been observed |
[77] |
|||||||||||||
TiO2NPs | Electrostatic interaction between TiO2 NPs and the bacterial cell surface results in suppression of cell division, degradation of the cell wall and cytoplasmic membrane due to the production of reactive oxygen species such as hydroxyl radicals and hydrogen peroxide | Penicillin G, amikacin, cephalexin, cefotaxime | MRSA | 10 mm increase in zone size. TiO2 nanoparticles significantly improved antibiotic efficacy against S. aureus when combined with beta-lactams, cephalosporins, and aminoglycosides |
[78] |
|||||||||||
Fe3O4NPs | Generation of reactive oxygen species from the disruption of the electronic transport chains owing to the resilient affinity of the iron-based nanoparticles for the cell membrane. Reactive oxygen species generated by Fe3O4 nanoparticles kill bacteria without harming non-bacterial cells | Streptomycin | S. aureus, E. coli, and P. aeruginosa | Zones of inhibition at concentrations (10, 20, 40, and 80): S. aureus (15 mm, 14 mm, 17 mm, 20 mm), E. coli (12 mm, 14 mm, 15 mm, 17 mm), P. aeruginosa (13 mm, 14 mm, 15 mm, 18 mm) | ||||||||||||
Kanamycin and rifampicin | E. coli and S. aureus | Kanamycin formed an inhibition zone against both, whereas rifampicin formed an inhibitory zone against S. aureus only |
[81] |
|||||||||||||
Amoxicillin | E. coli and S. aureus | A total of 9.9% and 8.9% increase in inhibitory effect observed in the presence of Cu NPs for E. coli and S. aureus, respectively |
[80] |
|||||||||||||
CuNPs | Generation of reactive oxygen species, lipid peroxidation, protein oxidation, and DNA degradation. Cu2+ ions released from nanoparticles penetrate bacterial cells and are subsequently oxidized intracellularly | Amikacin, ciprofloxacin, gentamicin, norfloxacin | E. coli, P. aeruginosa, Klebsiella spp. S. aureus | At 60 mg/mL, 18 mm for E. coli, 16 mm for Klebsiella |
[82] |
|||||||||||
BiNPs | Production of reactive oxygen species | Ciprofloxacin, norfloxacin, tetracycline, and metronidazole | K. pneumoniae | A synergistic effect was observed between all antibiotics and BiNPs. |
[83] |
|||||||||||
Cefotaxime, ampicillin, ceftriaxone, cefepime | E. coli, K. pneumoniae, | and P.aeruginosa | Significant decrease in MIC decrease with cefotaxime and ZnO NPs against K. pneumoniae (85.7%), P. aeruginosa (70%), and E. coli (50%) has been observed. Meanwhile, a decrease in MIC due to ZnO NP with other antibiotics has been observed. |
[84] |
||||||||||||
Norfloxacin, Ofloxacin, and Cephalexin | P. aeruginosa, E. coli | Significant increase in inhibition zone of antibiotics with ZnONPshave been observed against all isolates. |
[67] |
