Skin tissue engineering has made remarkable progress in wound healing treatment with the advent of newer fabrication strategies using natural/synthetic polymers and stem cells. Currently, stem cells and biomaterials are popularly used in the skin tissue engineering approach in different wound healing treatments. In skin tissue engineering application, stem cell facilitates in the regeneration of disintegrated tissue. Whereas, biomaterials serve as a platform to improve the engraftment of implanted cells and facilitate the function of exogenous cells by mimicking the tissue microenvironment. Hence, the combination and synergistic effect of biomaterials and stem cells have the potential to broaden the application of skin tissue engineering in wound healing treatment therapies.
- wound healing
- tissue engineering
- skin regeneration
1. Introduction
2. Skin Tissue Engineering and Regenerative Medicine
3. Techniques of Skin Tissue Engineering
4. Components of Skin Tissue Engineering and Regenerative Medicine
Tissue engineering and regenerative medicine (TERM) can be considered a multidisciplinary and emerging field in technology used to regenerate damaged organs, produce complex tissues, and maintain normal cell homeostasis [17][7]. TERM aims to design new tissues and organ replacements that closely mimic a typical physiological environment for cells. It has caused a revolution in the present and future therapeutic possibilities for acute and chronic wound healing, improving restoration of biological function and rehabilitation [20][10]. Advanced multidisciplinary TERM approaches involving growth factors, stem cells, and biomaterials are being adopted to induce regeneration or indirectly change the wound environment and stimulate healing [2]. The key components of regenerative medicine and tissue engineering (growth factors, cells, stem cells, biomaterials) (Figure 1) have unveiled several perspectives for skin tissue engineering and regeneration that can be used to address different stages of wound healing.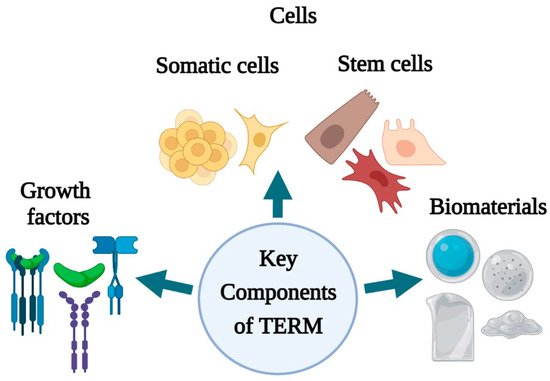
5. Conclusion
In summary, the use of growth factors, cells, and biomaterials have received attention in skin tissue engineering and regenerative medicine due to their ability and capacity to improve wound healing. However, immune sensitivity, compromised survival, proliferation, and differentiation of cells and, unable to fabricate a suitable scaffold limit the application of cells and biomaterials in clinical trials as well as in vitro and in vivo settings. With the aid of appropriate technology, these barriers can be overcome. Natural and synthetic biomaterials can be rationally designed for wound healing treatment according to their biophysical and biochemical properties. The incorporation of cells (both differentiated and stem cells) into structured and modified biomaterials increases the competence of restoring and repairing dysfunctional skin tissue and promotes wound healing parameters such as improved epithelialization, granulation tissue formation, vascularization, and angiogenesis. The well-organized spatial properties of a biomaterial or scaffold, in turn, can provide a protective and sometimes inducible microenvironment for the cells, mimicking the natural ECM. In addition, biomaterials are also being used to regulate stem cell fate before and after delivery by providing mechanical and biochemical support. Despite the encouraging results in non-clinical studies, only a handful of biomaterials have been used for skin tissue engineering in patients. Thus, additional clinical trials that use biomaterial should be performed to elucidate the influence of materials’ biophysical and biochemical properties on wound healing, tissue repair, and regeneration of humans. Hence, future efforts are essential to improve the clinical outcome in designing and fabricating biomaterials using emerging techniques like 3D bioprinting, electrospinning, and nanotechnology to meet specific properties of the components that need to be delivered for wound healing and regeneration.
References
- Ho, J.; Walsh, C.; Yue, D.; Dardik, A.; Cheema, U. Current advancements and strategies in tissue engineering for wound healing: A comprehensive review. Adv. Wound Care (New Rochelle) 2017, 6, 191–209.
- Tottoli, E.M.; Dorati, R.; Genta, I.; Chiesa, E.; Pisani, S.; Conti, B. Skin wound healing process and new emerging technologies for skin wound care and regeneration. Pharmaceutics 2020, 12, 735.
- Rezaie, F.; Momeni-Moghaddam, M.; Naderi-Meshkin, H. Regeneration and repair of skin wounds: Various strategies for treatment. Int. J. Low. Extrem. Wounds 2019, 18, 247–261.
- Gurtner, G.C.; Chapman, M.A. Regenerative medicine: Charting a new course in wound healing. Adv. Wound Care 2016, 5, 314–328.
- Xu, Y.; Chen, C.; Hellwarth, P.B.; Bao, X. Biomaterials for stem cell engineering and biomanufacturing. Bioact. Mater. 2019, 4, 366–379.
- Chaudhari, A.A.; Vig, K.; Baganizi, D.R.; Sahu, R.; Dixit, S.; Dennis, V.; Singh, S.R.; Pillai, S.R. Future prospects for scaffolding methods and biomaterials in skin tissue engineering: A review. Int. J. Mol. Sci. 2016, 17, 1974.
- Boyce, S.T.; Lalley, A.L. Tissue engineering of skin and regenerative medicine for wound care. Burn. Trauma 2018, 6, 4.
- Dhasmana, A.; Singh, S.; Kadian, S.; Singh, L. Skin tissue engineering: Principles and advances. J. Dermatol. Skin 2018, 1, 3–6.
- Nicholas, M.N.; Jeschke, M.G.; Amini-Nik, S. Methodologies in creating skin substitutes. Cell. Mol. Life Sci. 2016, 73, 3453–3472.
- Gomes, M.E.; Rodrigues, M.T.; Domingues, R.M.A.; Reis, R.L. Tissue engineering and regenerative medicine: New trends and directions-A year in review. Tissue Eng. Part B Rev. 2017, 23, 211–224.
- Nourian Dehkordi, A.; Mirahmadi Babaheydari, F.; Chehelgerdi, M.; Raeisi Dehkordi, S. Skin tissue engineering: Wound healing based on stem-cell-based therapeutic strategies. Stem Cell Res. Ther. 2019, 10, 111.
- Nakayama, C.; Fujita, Y.; Matsumura, W.; Ujiie, I.; Takashima, S.; Shinkuma, S.; Nomura, T.; Abe, R.; Shimizu, H. The development of induced pluripotent stem cell-derived mesenchymal stem/stromal cells from normal human and RDEB epidermal keratinocytes. J. Dermatol. Sci. 2018, 91, 301–310.
- Blackstone, B.N.; Hahn, J.M.; McFarland, K.L.; DeBruler, D.M.; Supp, D.M.; Powell, H.M. Inflammatory response and biomechanical properties of coaxial scaffolds for engineered skin in vitro and post-grafting. Acta Biomater. 2018, 80, 247–257.
- Roseti, L.; Parisi, V.; Petretta, M.; Cavallo, C.; Desando, G.; Bartolotti, I.; Grigolo, B. Scaffolds for bone tissue engineering: State of the art and new perspectives. Mater. Sci. Eng. C 2017, 78, 1246–1262.
- Eltom, A.; Zhong, G.; Muhammad, A. Scaffold techniques and designs in tissue engineering functions and purposes: A Review. Adv. Mater. Sci. Eng. 2019, 3429527.
- Raeisdasteh Hokmabad, V.; Davaran, S.; Ramazani, A.; Salehi, R. Design and fabrication of porous biodegradable scaffolds: A strategy for tissue engineering. J. Biomater. Sci. Polym. Ed. 2017, 28, 1797–1825.
- Mondschein, R.J.; Kanitkar, A.; Williams, C.B.; Verbridge, S.S.; Long, T.E. Polymer structure-property requirements for stereolithographic 3D printing of soft tissue engineering scaffolds. Biomaterials 2017, 140, 170–188.
- Tarassoli, S.P.; Jessop, Z.M.; Al-Sabah, A.; Gao, N.; Whitaker, S.; Doak, S.; Whitaker, I.S. Skin tissue engineering using 3D bioprinting: An evolving research field. J. Plast. Reconstr. Aesthetic. Surg. 2018, 71, 615–623.
- Ramanathan, G.; Singaravelu, S.; Muthukumar, T.; Thyagarajan, S.; Perumal, P.T.; Sivagnanam, U.T. Design and characterization of 3D hybrid collagen matrixes as a dermal substitute in skin tissue engineering. Mater. Sci. Eng. C 2017, 72, 359–370.
- Park, Y.R.; Ju, H.W.; Lee, J.M.; Kim, D.-K.; Lee, O.J.; Moon, B.M.; Park, H.J.; Jeong, J.Y.; Yeon, Y.K.; Park, C.H. Three-dimensional electrospun silk-fibroin nanofiber for skin tissue engineering. Int. J. Biol. Macromol. 2016, 93, 1567–1574.
- Choi, S.M.; Lee, K.M.; Kim, H.J.; Park, I.K.; Kang, H.J.; Shin, H.C.; Baek, D.; Choi, Y.; Park, K.H.; Lee, J.W. Effects of structurally stabilized EGF and bFGF on wound healing in type I and type II diabetic mice. Acta Biomater. 2018, 66, 325–334.
- Li, M.; Qiu, L.; Hu, W.; Deng, X.; Xu, H.; Cao, Y.; Xiao, Z.; Peng, L.; Johnson, S.; Alexey, L.; et al. Genetically-modified bone mesenchymal stem cells with TGF-beta3 improve wound healing and reduce scar tissue formation in a rabbit model. Exp. Cell Res. 2018, 367, 24–29.
- Jeong, S.; Kim, B.; Park, M.; Ban, E.; Lee, S.H.; Kim, A. Improved diabetic wound healing by EGF encapsulation in gelatin-alginate coacervates. Pharmaceutics 2020, 12, 334.
- Xu, K.; An, N.; Zhang, H.; Zhang, Q.; Zhang, K.; Hu, X.; Wu, Y.; Wu, F.; Xiao, J.; Zhang, H.; et al. Sustained-release of PDGF from PLGA microsphere embedded thermo-sensitive hydrogel promoting wound healing by inhibiting autophagy. J. Drug. Deliv. Technol. 2020, 55, 101405.
- Chen, G.; Yu, Y.; Wu, X.; Wang, G.; Ren, J.; Zhao, Y. Bioinspired multifunctional hybrid hydrogel promotes wound healing. Adv. Funct. Mater. 2018, 28, 1801386.
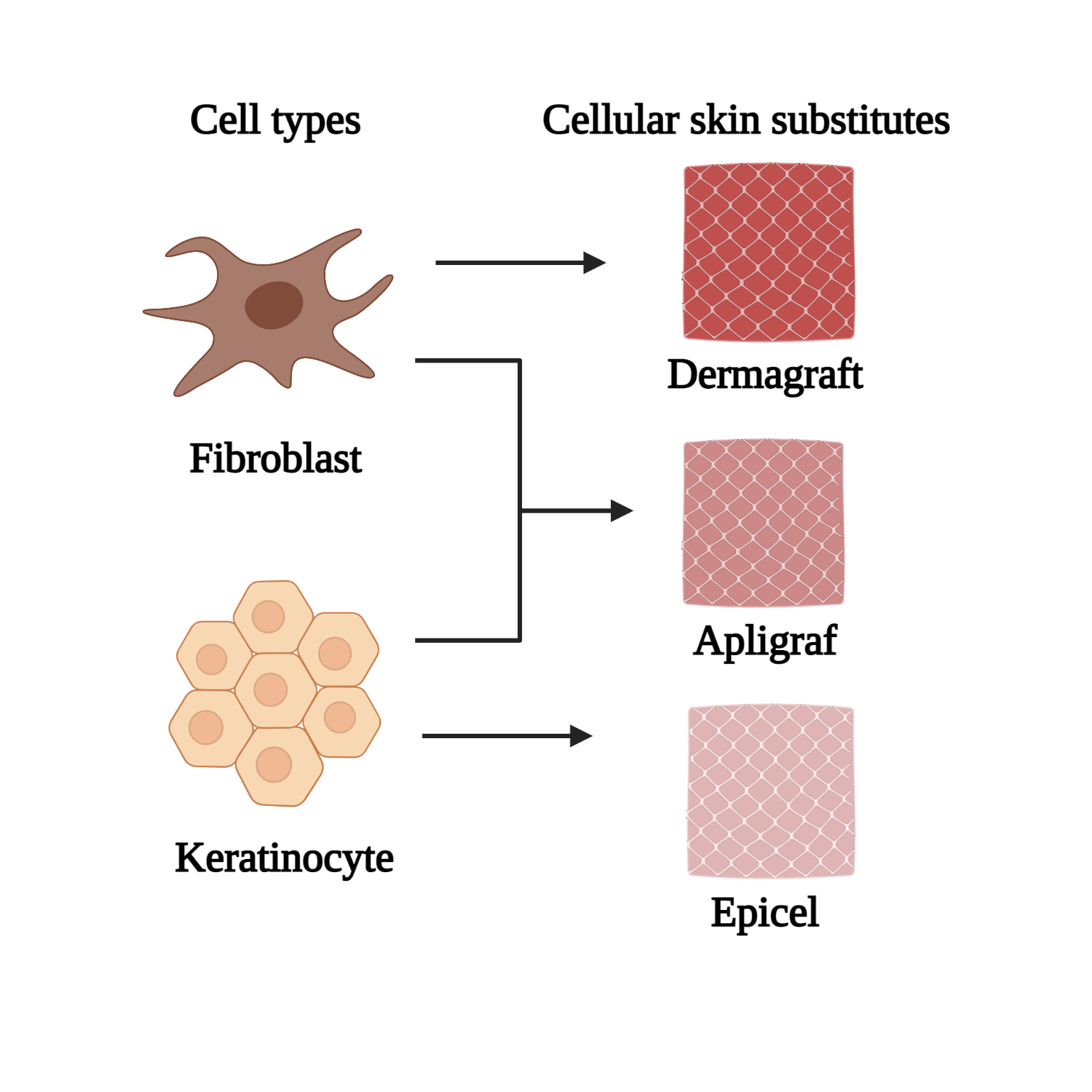
- Kallis, P.J.; Friedman, A.J.; Lev-Tov, H. A guide to tissue-engineered skin substitutes. J. Drugs Dermatol. 2018, 17, 57–64.
- Ter Horst, B.; Chouhan, G.; Moiemen, N.S.; Grover, L.M. Advances in keratinocyte delivery in burn wound care. Adv. Drug Deliv. Rev. 2018, 123, 18–32.
- Nicholas, M.N.; Yeung, J. Current status and future of skin substitutes for chronic wound healing. J. Cutan. Med. Surg. 2017, 21, 23–30.
- Tavakoli, S.; Klar, A.S. Bioengineered skin substitutes: Advances and future trends. Appl. Sci. 2021, 11, 1493.
- Chang, D.K.; Louis, M.R.; Gimenez, A.; Reece, E.M. The basics of integra dermal regeneration template and its expanding clinical applications. Semin. Plast. Surg. 2019, 33, 185–189.
- Duscher, D.; Barrera, J.; Wong, V.W.; Maan, Z.N.; Whittam, A.J.; Januszyk, M.; Gurtner, G.C. Stem cells in wound healing: The future of regenerative medicine? A mini-review. Gerontology 2016, 62, 216–225.
- Kucharzewski, M.; Rojczyk, E.; Wilemska-Kucharzewska, K.; Wilk, R.; Hudecki, J.; Los, M.J. Novel trends in application of stem cells in skin wound healing. Eur. J. Pharmacol. 2019, 843, 307–315.
- Dash, B.C.; Xu, Z.; Lin, L.; Koo, A.; Ndon, S.; Berthiaume, F.; Dardik, A.; Hsia, H. Stem cells and engineered scaffolds for regenerative wound healing. Bioengineering 2018, 5, 23.
- Aramwit, P. Introduction to biomaterials for wound healing. In Wound Healing Biomaterials; Agren, M.S., Ed.; Woodhead Publishing: Cambridge, UK, 2016; pp. 3–38.
- Chen, F.M.; Liu, X. Advancing biomaterials of human origin for tissue engineering. Prog. Polym. Sci. 2016, 53, 86–168.
- Sheikholeslam, M.; Wright, M.E.E.; Jeschke, M.G.; Amini-Nik, S. Biomaterials for skin substitutes. Adv. Healthc. Mater. 2018, 7, 1700897.
