Tissue engineering and regenerative medicine (TERM) can be considered a multidisciplinary and emerging field in technology used to regenerate damaged organs, produce complex tissues, and maintain normal cell homeostasis
. TERM aims to design new tissues and organ replacements that closely mimic a typical physiological environment for cells. It has caused a revolution in the present and future therapeutic possibilities for acute and chronic wound healing, improving restoration of biological function and rehabilitation
. Advanced multidisciplinary TERM approaches involving growth factors, stem cells, and biomaterials are being adopted to induce regeneration or indirectly change the wound environment and stimulate healing
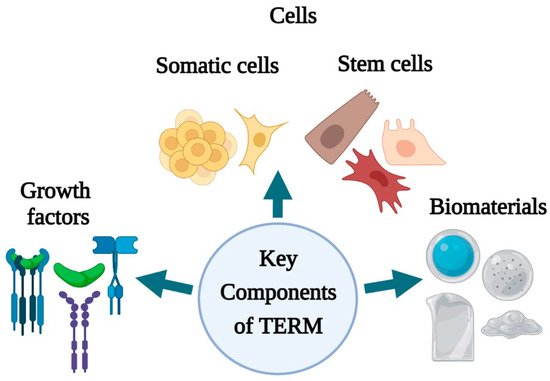
. The key components of regenerative medicine and tissue engineering (growth factors, cells, stem cells, biomaterials) () have unveiled several perspectives for skin tissue engineering and regeneration that can be used to address different stages of wound healing.
Figure 1.
Key components of skin tissue engineering and regenerative medicine (created with BioRender.com).
Growth factors (GFs) are defined as the biologically active polypeptides that control tissue repair via interacting with specific cell surface receptors. They play a prominent role in cell migration into the wound area, promote epithelialization, initiate angiogenesis, and stimulate matrix formation followed by remodeling the wounded area
[11]. Epidermal growth factor (EGF)
[23][41], basic fibroblast growth factor (bFGF)
[21][39], transforming growth factor-beta (TGFβ
3)
[22][40], platelet-derived growth factor (PDGF)
[24][42], and vascular endothelial growth factor (VEGF)
[25][43] are some of the GFs that contribute to the wound-healing process. Several researchers have proven the role of each growth factor in wound healing by promoting angiogenesis, cell migration, re-epithelialization, and granulation tissue formation. Moreover, a handful of studies verified the potential of using growth factors in combination with carriers for effective delivery in maximizing wound healing.
Apart from growth factors, both cells and cellular skin substitutes (both differentiated and stem cells) have exhibited great potential by providing all the elements required for skin regeneration such as cells, mediators, and materials mimicking ECM
[3]. The use of viable cells cultured in special conditions are used to produce cell sheet substitutes that contribute to wound repair. Among the available differentiated human cells, fibroblasts and keratinocytes are the primary sources for epidermal and dermal substitute production, respectively
[26][49]. Taking advantage of the wound healing properties of fibroblasts and keratinocytes, specific cell composition constructs have been developed according to the treatment target for dermal and epidermal regeneration
[2]. When applied to the wounded area, the cells supply signaling molecules, growth factors, and ECM proteins that aid healing
[27][52]. Through a paracrine crosstalk mechanism, fibroblasts and keratinocytes communicate with each other, leading to cell recruitments and maintenance of skin homeostasis, which is desirable for complete wound healing. For this purpose, several double-layer dermal cellular skin substitutes have been synthesized commercially, incorporating both fibroblasts and keratinocytes for the repair and regeneration of chronic wounds
[28][53]. EpiCel, Dermagraft, and Apligraf (
Figure 2) are some of the instances of commercially available cellular skin substitute products that incorporate both keratinocytes and fibroblasts, respectively. These novel products are assembled according to their specific conformation and structure; in particular, pore sizes and their distribution are essential in providing a suitable matrix for effective cell migration and arrangement
[29][54]. The novelty of these products represents a basis for revascularization, forming a proper microenvironment for cellular migration and proliferation
[30][55].
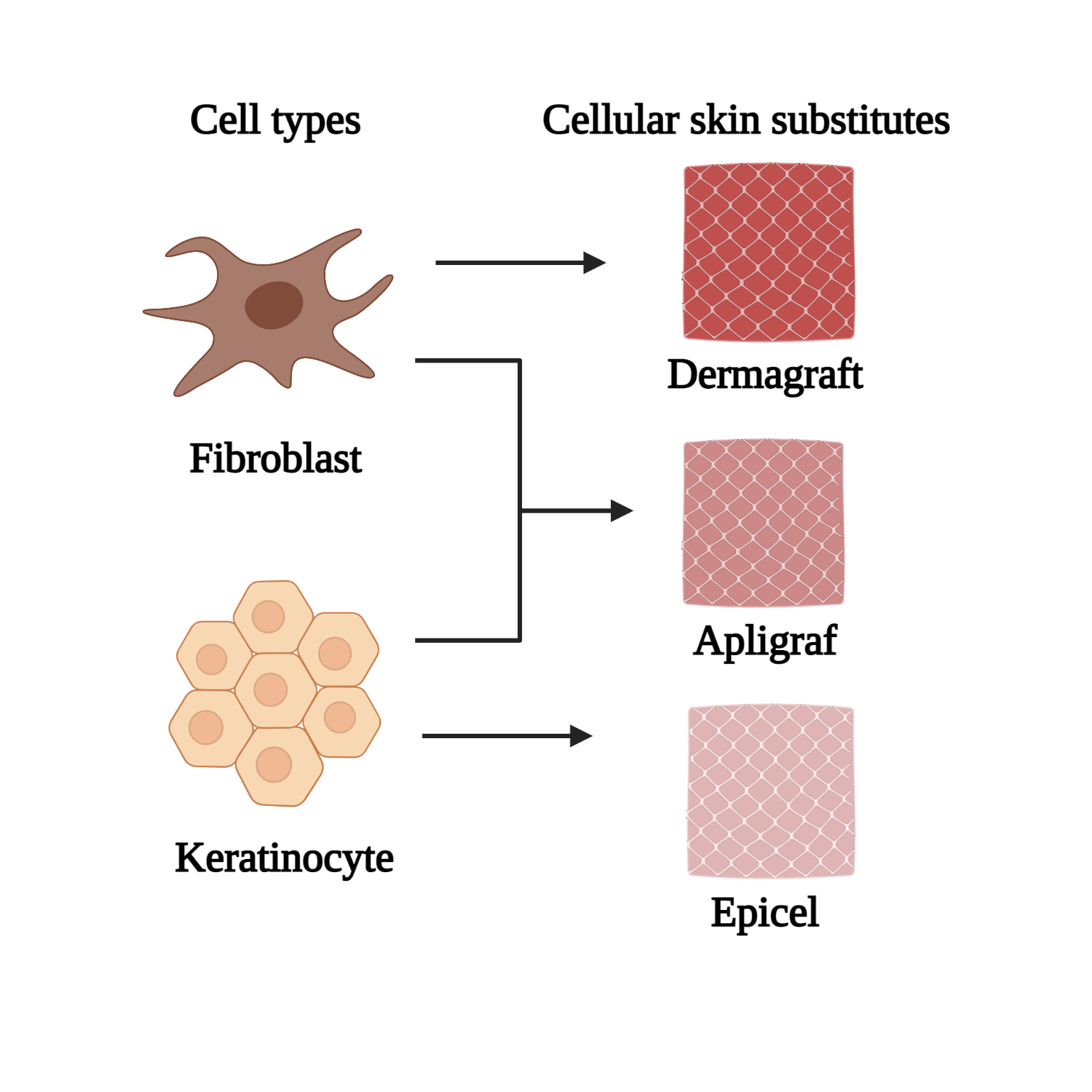
Figure 2.
Cellular skin substitutes made from fibroblast and keratinocyte (created with BioRender.com)
The use of stem cells (SC) has become a promising new approach in tissue engineering and regenerative medicine for skin injury treatment. Stem cells can be defined and characterized based on their capacity for self-renewal, asymmetric replication, and potency
[31][56]. They are attributed with the ability to replenish lost cells throughout the lifespan of an organism via unlimited replication, providing a population of sister stem cells
[32][57]. The main clinical focus of stem cell application in wound healing is to accelerate the healing process, prevent wound contracture and scar formation, and initiate earlier wound closure and regeneration of skin and its appendages. Besides, stem cells can secrete pro-regenerative cytokines, making them an attractive agent for treating chronic wounds
[31][56]. Among the primary sources of cells that are in use for wound healing and the regeneration of injured skin are embryonic stem cells (ESCs), adult stem cells, and induced pluripotent stem cells (iPSCs)
[33][58]In tissue engineering, biomaterials play a prominent role in unlocking the regenerative potential innate to human tissues/organs, restoring deteriorated states, and re-establishing normal bodily function
[34][76]. Biomaterial science and engineering have witnessed tremendous growth in the past five decades due to vast investment in developing new products
[35][77]. In a broader sense, biomaterials can be defined as material devices or implants used to repair or replace native body tissues or as a provisional scaffolding material adopted to construct human-made tissues or organs
[34][76]. Using biomaterial in tissue engineering aims to provide temporary mechanical support and mass transport to encourage cell adhesion, proliferation, migration, and differentiation and control the size and shape of the regenerated tissue
[36][78]. Moreover, biomaterials known as temporary scaffolds act as an ECM template that is actively involved in delivering cues to the cells that form the regenerated tissues
[4]. Numerous approaches are adopted for designing matrices, comprised of innovative biomaterials possessing two crucial traits: Biocompatibility (the materials must hold minimal toxicity and immunogenicity) and biodegradability (the materials must be easily removable upon completion of their function)
[36][78]. Furthermore, they must also possess the ability to interact with a biological environment and particularly modulate cellular response
[4]. Hence, biomaterials have become an active part of cellular function regulation and act as a support for tissue regeneration or a platform for drug delivery.
5. Conclusion
In summary, the use of growth factors, cells, and biomaterials have received attention in skin tissue engineering and regenerative medicine due to their ability and capacity to improve wound healing. However, immune sensitivity, compromised survival, proliferation, and differentiation of cells and, unable to fabricate a suitable scaffold limit the application of cells and biomaterials in clinical trials as well as in vitro and in vivo settings. With the aid of appropriate technology, these barriers can be overcome. Natural and synthetic biomaterials can be rationally designed for wound healing treatment according to their biophysical and biochemical properties. The incorporation of cells (both differentiated and stem cells) into structured and modified biomaterials increases the competence of restoring and repairing dysfunctional skin tissue and promotes wound healing parameters such as improved epithelialization, granulation tissue formation, vascularization, and angiogenesis. The well-organized spatial properties of a biomaterial or scaffold, in turn, can provide a protective and sometimes inducible microenvironment for the cells, mimicking the natural ECM. In addition, biomaterials are also being used to regulate stem cell fate before and after delivery by providing mechanical and biochemical support. Despite the encouraging results in non-clinical studies, only a handful of biomaterials have been used for skin tissue engineering in patients. Thus, additional clinical trials that use biomaterial should be performed to elucidate the influence of materials’ biophysical and biochemical properties on wound healing, tissue repair, and regeneration of humans. Hence, future efforts are essential to improve the clinical outcome in designing and fabricating biomaterials using emerging techniques like 3D bioprinting, electrospinning, and nanotechnology to meet specific properties of the components that need to be delivered for wound healing and regeneration.