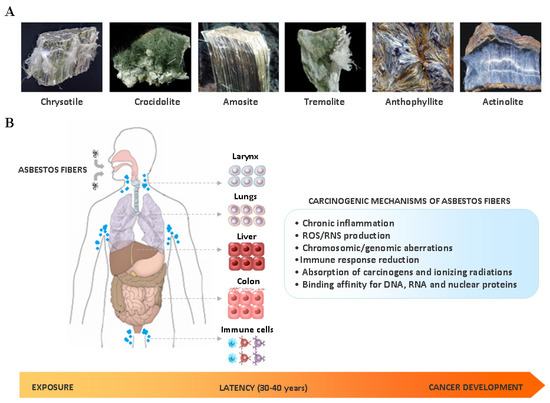
The link between asbestos exposure and the onset of thoracic malignancies is well established. However epidemiological studies have provided evidences that asbestos may be also involved in the development of gastrointestinal tumors, including intrahepatic cholangiocarcinoma (ICC). In line with this observation, asbestos fibers have been detected in the liver of patients with ICC. Although the exact mechanism still remains unknown, the presence of asbestos fibers in the liver could be explained in the light of their translocation pathway following ingestion/inhalation. In the liver, thin and long asbestos fibers could remain trapped in the smaller bile ducts, particularly in the stem cell niche of the canals of Hering, and exerting their carcinogenic effect for a long time, thus inducing hepatic stem/progenitor cells (HpSCs) malignant transformation. In this scenario, chronic liver damage induced by asbestos fibers over the years could be seen as a classic model of stem cell-derived carcinogenesis, where HpSC malignant transformation represents the first step of this process. This phenomenon could explain the recent epidemiological findings, where asbestos exposure seems mainly involved in ICC, rather than extrahepatic cholangiocarcinoma, development.
- intrahepatic cholangiocarcinoma
- asbestos
- hepatic stem/progenitor cells
1. Introduction
| Risk Factor | Association with ICC |
|---|---|
| Bile duct cysts/Caroli’s disease | very strong |
| Primary sclerosing cholangitis/cholangitis | very strong * |
| Hepatolithiasis | strong/very strong |
| Cholelithiasis/choledocholithiasis | moderate/strong |
| Cirrhosis | strong/very strong |
| HBV/HCV infection | moderate/strong |
| Hemochromatosis | moderate |
| Inflammatory bowel disease/chronic pancreatitis | moderate |
| Duodenal/gastric ulcer | weak/modest |
| O. viverrini/C. sinensis infection | strong * |
| Diabete type II | weak/modest |
| Obesity | weak/modest * |
| NAFLD/NASH | strong |
| Alcohol | moderate |
| Cigarette smoking | weak/modest |
| Thorotrast | very strong * |
| 1,2-dichloropropane | very strong * |
2. Asbestos Carcinogenesis
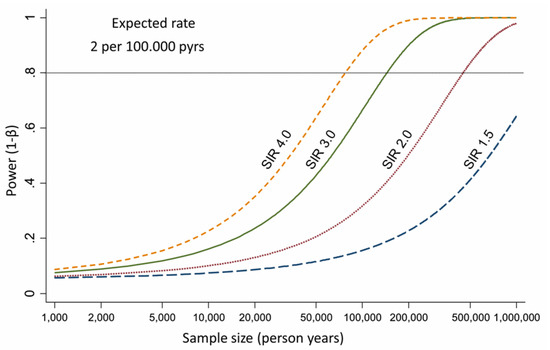
3. Epidemiological Evidences about Asbestos Exposure and ICC Development
| Reference | Period | Cohort | Workers’ Category | SMR or SIR * (95% CI) |
Tumor Site |
|---|---|---|---|---|---|
| Selikoff I. et al., 1991 [34] | 1967–1987 | 17800 (M) | Insulator workers | 1.08 2.61 |
Liver Bile ducts + Gallbladder |
| Battista G. 1999 [35] | 1945–1970 | 734 (M) | Railway workers | 241 (126–420) | Liver |
| Berry G. et al., 2000 [36] | 1933–1980 | 5000 (M/F) | Factory workers | 2.66 (1.28–4.89) | Liver + Bile ducts + Gallbladder |
| Wingren G. 2004 [37] | 1964–1997 | 1229 (M/F) | Art glassworks | * 2.00 (0.41–5.84) (M) * 4.35 (0,75–10.59) (F) |
Liver + Bile ducts |
| Hein MJ. et al., 2007 [38] | 1940–2001 | 3072 (M/F) | Textile workers | 1.05 (0.51–1.94) | Liver + Biliary tract |
| Pira E. et al., 2007 [39] | 1946–1984 | 1966 (M/F) | Textile workers | 237 (118–425) | Liver |
| Clin B. et al., 2009 [40] | 1978–2004 | 2024 (M/F) | Textile workers | * 1.61 (0.86–2.75) * 1.92 (0.38–5.6) |
Liver Biliary tract |
| Wang X. et al., 2013 [41] | 1972–2008 | 586 (M) 272 (F) |
Textile workers | 1.34 (0.81–2.21) – |
Liver + Bile duct |
| Hogstedt T. et al., 2013 [42] | 1958–2006 | 6320 (M/F) | Chimney sweeps | * 2.48 (1.47–3.91) * 1.6 (0.19–5.78) |
Liver Bile ducts |
| Boulanger M. et al., 2015 [43] | 1978–2009 | 2024 (M/F) | Textile workers | * 1.85 (1.09–2.92) (M) * 2.84 (0.76–7.26) (M) |
Liver Biliary tract |
| * 1.85 (1.09–2.92) (F) * 2.84 (0.76–7.26) (F) |
Liver Biliary tract |
||||
| Wu W. et al., 2015 [44] | 1975–1989 | 4427 (M/F) | Shipbreaking workers | 1.6 (1.08–2.36) | Liver + Intrahepatic bile ducts |
| Pira E. et al., 2016 [45] | 1946–2013 | 1977 (M/F) | Textile workers | 1.06 (0.55–1.86) | Liver |
| Pira E. et al., 2017 [46] | 1946–2014 | 1056 (M) | Miners | 0,65 (0.21–1.51) | Liver |
| Luberto F. et al., 2019 [47] | 1934–2006 | 13076 (M/F) | Cement workers | 0.99 (0.81–1.20) (M) 0.84 (0.42–1.60) (F) |
Liver + Intrahepatic bile ducts |
