Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Patrizia Limonta and Version 2 by Vivi Li.
Prostate cancer (PCa) represents a major cause of cancer mortality among men in developed countries. Patients with recurrent disease initially respond to androgen-deprivation therapy, but the tumor eventually progresses into castration-resistant PCa; in this condition, tumor cells acquire the ability to escape cell death and develop resistance to current therapies. Thus, new therapeutic approaches for PCa management are urgently needed. In this setting, natural products have been extensively studied for their anti-PCa activities, such as tumor growth suppression, cell death induction, and inhibition of metastasis and angiogenesis.
- prostate cancer
- natural compounds
- phytochemicals
- chemoprevention
- novel therapeutic strategies
1. Introduction
Globally, prostate cancer (PCa) is the most frequently diagnosed tumor in men, being particularly common in Western countries [1]. In about 90% of cases, PCa is still organ-confined or only locally advanced at diagnosis, which makes it effectively treatable with prostatectomy or local radiotherapy. However, 30–40% of patients usually experience progression of disease [2]; at this stage, where tumor growth depends on androgens, the most effective treatment is represented by androgen-deprivation therapy, aimed at blocking hormone secretion and/or activity. This therapy is based on pharmacological castration, obtained by administration of GnRH agonists, alone or in combination with antiandrogens [3][4][3,4]; more recently, two major clinical trials, CHAARTED and STAMPEDE, have also demonstrated benefits of early initiation of chemotherapy concomitantly with hormonal therapy [5][6][5,6]. However, despite a good initial response, relapse occurs in the majority of patients within 2–3 years, and the tumor progresses towards a condition of resistance to castration [7]. Improved therapeutic options for castration-resistant patients are needed, since taxane-based (i.e., docetaxel) treatment and immunotherapy, as well as the novel therapies with enzalutamide and abiraterone, generally offer a progression-free survival of a few months [8][9][8,9]. Parallelly, bone metastases, occurring in 80% of advanced PCas and usually treated with radiation therapy and chemotherapy, are associated with considerable morbidity, adversely affect quality of life and several skeletal-related events [4][10][4,10]. Therefore, in the last years natural compounds have gained a lot of interest, due to their various anti-cancer effects. In fact, accumulating evidence has highlighted that nutraceuticals can exert growth-suppressing, pro-death, anti-metastatic, and anti-angiogenic activity in PCa cell lines and xenografts, while sparing normal prostate epithelial cells [11]. In particular, several mechanisms are involved in the anti-PCa actions of these molecules, including inhibition of androgen receptor (AR) axis and targeting of cancer stemness [12][13][12,13].
2. Natural Compounds with Potential to Treat Prostate Cancer
Data from literature have pointed out that several natural products can selectively target numerous molecules and signaling pathways implicated in tumor development and progression [11][12][13][11,12,13]. Many of them have been tested in in vitro and in vivo studies, while some clinical trials have been conducted or are currently ongoing [11][12][13][11,12,13]. Among these naturally occurring molecules, quercetin, fisetin, luteolin, apigenin, curcumin, resveratrol, genistein, silibinin, kaempferol, epigallocatechin-3-gallate (EGCG), tocotrienols, sulforaphane, ginsenosides, ursolic acid, berberine, honokiol, xanthoumol, oridonin, and tannic acid have shown outstanding potential as anti-PCa agents in in vitro and preclinical experiments (Figure 1).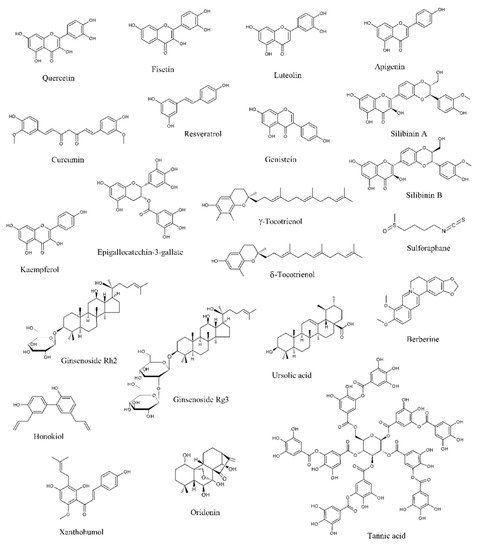
Figure 1. Chemical structures of the major anti-prostate cancer (PCa) phytochemicals.
2.1. Natural Compounds Modulating the Androgen Receptor Axis
A number of studies indicates that PCa growth and progression are driven by the AR, a ligand-dependent transcription factor and member of the nuclear receptor family [14]. The AR is encoded by the AR gene located on the X chromosome at Xq11-12 and displays a N-terminal regulatory domain, a DNA-binding domain (DBD), a ligand-binding domain (LBD), and a C-terminal domain. In the absence of androgens, particularly dihydrotestosterone (DHT) and testosterone, it is complexed with chaperone proteins, heat-shock protein 90 (Hsp90) and 70 (Hsp70), in the cell cytoplasm. Upon ligand binding, it is transferred to the nucleus, where it homodimerizes due to the interactions of dedicated motifs in the DBD and in the LBD. Then, the dimerized receptor recognizes cognate DNA response elements in regulatory regions located in proximal or more distal intra- and inter-genic regions of androgen target genes [15][16][15,16]. It then recruits different coregulator proteins and epigenetic factors to generate a transcriptionally active complex able to upregulate downstream pro-survival gene expression [14].
Given its fundamental role in PCa cell proliferation, the AR signaling represents a crucial target for PCa management. In this context, pharmacological castration obtained via androgen-deprivation therapy is currently the most effective strategy for PCa treatment. However, PCa often becomes castration resistant [8][9][8,9]. One of the mechanisms underlying this change is an enhanced AR expression in the tumor cell. In particular, it has been shown that 28% of cancers resistant to androgen-deprivation therapy display AR upregulation due to amplification of its gene [17]. Another mechanism responsible for PCa androgen-independent growth is ligand promiscuity, caused by mutations of the AR gene that lead to amino acid substitutions in the LBD and subsequent decrease in the specificity and selectivity for ligands: the most common of them are T877A, F876L, W741L, and L701H. These mutant AR proteins bind to other steroids, including progesterone, estrogens, and glucocorticoids, which can activate the AR signaling pathway and promote PCa progression [18]. AR activation via ligand-independent mechanisms represents the third mechanism of androgen-independent PCa development [19]. Indeed, it has been found that tyrosine kinase receptor-activating ligands, such as epidermal growth factor (EGF) and insulin-like growth-factor-1 (IGF-1), can activate the AR through the phosphoinositide 3-kinase (PI3K)/Akt/mammalian target of rapamycin (mTOR) pathway [20][21][22][23][24][20,21,22,23,24]. Finally, various AR splice variants lacking the LBD have been recently reported: the AR N-terminal domain becomes constitutively active in the absence of the LBD, thereby promoting castration resistant proliferation [25][26][25,26].
Interestingly, various phytochemicals have been shown to modulate AR expression and activity.
Quercetin is a penta-hydroxylated flavonol, naturally occurring in tea, onions, apples, tomatoes, and capers and endowed with important chemopreventive and anti-cancer properties [27]. Yuan et al. demonstrated that in LNCaP PCa cells a protein complex containing the AR, specific protein 1 (Sp1) and c-Jun was generated in response to quercetin treatment and suppressed AR function. This resulted in the inhibition of the production of the prostate-specific, androgen-related tumor markers prostate-specific antigen (PSA) and human kallikrein-2 (hK2), as well as in the downregulation of androgen-related genes, such as ornithine decarboxylase (ODC) and NKX3.1 [28][29][30][31][28,29,30,31]. Interestingly, quercetin was also able to repress the expression of the AR splice variant 7 (AR-V7), which correlates to resistance to enzalutamide and poor prognosis, via Hsp70 inhibition [32].
Fisetin, a flavonol present in strawberries, apples, persimmons, onions, kiwi, and cucumbers, has been recently demonstrated to exert not only potent neuroprotective effects but also different anti-tumor activities [33][34][33,34]. In PCa, it was shown to specifically bind to the AR LBD. This interaction resulted in a decreased AR stability and amino-terminal/carboxyl-terminal (N-C) interaction, leading to a reduced transactivation of AR target genes. Moreover, fisetin treatment of LNCaP cells was followed by a downregulation of AR levels, due to a reduction in its promoter activity and to an increase of its degradation. In this cell line, the flavonol also synergized with bicalutamide in promoting apoptotic cell death. Finally, in AR-positive CWR22υ1 PCa cell-bearing mice, fisetin inhibited tumor growth and decreased PSA serum levels, suggesting that this compound is able to suppress AR activity also in vivo [35].
Luteolin, a flavone abundant in rosemary, thyme, parsley, broccoli, and celery, is characterized by anti-inflammatory, neuroprotective, and anti-cancer activity [36][37][36,37]. It was observed to induce a dose- and time-dependent decrease in AR mRNA and protein expression, as well as of intracellular and secreted PSA levels, in PCa cells. In particular, it appears to promote the AR-Hsp90 complex dissociation, causing AR degradation via the proteasome-ubiquitin pathway [38].
Curcumin is a polyphenol extracted from turmeric (Curcuma longa), which has shown great therapeutic potential [39][40][41][39,40,41]. This compound was demonstrated not only to decrease the expression of AR and AR-related cofactors, such as activator protein-1 (AP-1), nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB), CREB-binding protein (CBP), and NKX3.1, but also to reduce testosterone production in PCa cell lines and xenografts. This reduction in testosterone levels was associated with a downregulation of steroidogenic acute regulatory proteins, including cytochrome P450 11A1 (CYP11A1) and 3-beta-hydroxysteroid dehydrogenase 2 (HSD3B2), and in an enhanced expression of aldo-keto reductase 1C2 (AKR1C2), a 3-ketosteroid reductase responsible for the elimination of 5alpha-DHT and subsequent inactivation of AR [42][43][44][45][42,43,44,45].
Resveratrol is a grape-derived polyphenol that possesses numerous health benefits, including various chemopreventive effects [46]. It was found to target the AR axis in different in vitro and in vivo PCa models [47][48][49][50][51][47,48,49,50,51]. On one hand, in LNCaP cells it inhibited β-catenin nuclear translocation through hypoxia-inducible factor 1-α (HIF-1α) downregulation, thus suppressing β-catenin-mediated AR signaling [52]; similarly, it also repressed interleukin-6 (IL-6)-induced AR transcriptional activity [53]. On the other hand, in 22RV1 cells it promoted the AR splice variant ARV7 proteasomal degradation, by enhancing its polyubiquitination. These data indicate that resveratrol could be used not only for the treatment of androgen-responsive PCa but also for the management of the ARV7-positive castration-resistant tumor [54].
Genistein is a common phytoestrogen that can be obtained from soybeans [55]. Indeed, it was shown to inhibit the AR signaling via estrogen receptor-β (ER-β) and estrogen-related pathways, as well as through suppression of Akt/Forkhead box O3a (FOXO3a)/glycogen synthase kinase 3β (GSK-3β) and histone deacetylase 6 (HDAC6)-Hsp90 function, needed to stabilize the AR [56][57][58][59][56,57,58,59]. Notably, in a recent study by Mahmoud et al., genistein was also demonstrated to bind to both the wild and the T877A-mutant types of AR, specifically competing with androgens. In particular, while it suppressed proliferation of AR wild-type LAPC-4 cells, it exerted a dual role in T877A-mutated LNCaP and PC3 cell lines, by stimulating cell growth at lower doses and inducing cell death at higher concentrations [60]. Finally, in PCa cells genistein downregulated prostate androgen-regulated transcript-1 (PART-1) gene expression induced by DHT, thus affecting cell proliferation [61].
Other natural products that have been demonstrated to trigger similar inhibitory effects on the AR axis are sulforaphane [62][63][64][65][62,63,64,65], epigallocatechin-3-gallate (EGCG) [66][67][66,67], ginsenosides [68][69][70][71][68,69,70,71], silymarin [72], berberine [73], honokiol [74], and celastrol [75].
2.2. Natural Compounds Affecting Proliferation
Numerous natural compounds have been reported to exert growth-suppressive and anti-proliferative activities in PCa cells and xenografts.
Epidermal growth factor receptor (EGFR) is a receptor tyrosine kinase whose activation is associated with an increase in cell growth and survival, which explains why EGFR is commonly overexpressed/overactivated in tumors of epithelial origin, including PCa. In particular, after binding to its specific ligands, such as EGF and transforming growth factor α (TGFα), it triggers several downstream signaling pathways, including PI3K/Akt/mTOR, mitogen-activated protein kinases (MAPKs), Hedgehog (Hh) signaling, and NF-κB [76]. Many phytochemicals, including quercetin, luteolin, resveratrol, genistein, and berberine, have been shown to reduce EGFR levels, as well as to suppress its intrinsic tyrosine kinase activity and its ligand-induced activation, in different PCa cell lines and in vivo models [77][78][79][80][81][77,78,79,80,81].
The IGF axis is a complex signaling network implicated in different tumorigenic processes, particularly in cancer proliferation, survival, and metabolism. It involves the interaction between the peptide-ligands IGF1 and IGF2 and the receptors IGF1R and IGF2R, and its activation elicits downstream signals, such as the PI3K/AKT and the MAPK pathways [82]. Interestingly, the IGF axis represents a major target for the anti-PCa action of silibinin, a flavonoid endowed with antioxidant properties commonly found in the milk thistle (Silybum marianum) [83][84][83,84]. Indeed, it decreased IGF1 expression and increased IGFBP-3 levels in transgenic adenocarcinoma of the mouse prostate (TRAMP) models, thus inhibiting tumor growth and progression [85][86][87][85,86,87]. Similar results were also obtained after treatment of PCa-bearing mice with luteolin [88].
Emerging evidence has highlighted the key role played by the PI3K/AKT pathway in the development of castration resistant PCa. This cascade, which is activated in most of advanced PCas, acts as a fundamental driver for tumor cell proliferation, thereby allowing cancer cells to survive to the androgen deprivation-related cytotoxicity. Moreover, preclinical studies have highlighted a strict correlation between the PI3K/AKT and AR axes, evidencing a dynamic cross-talk between these cascades in the acquisition of androgen-deprivation therapy resistance. Therefore, there is an evident rationale for the development of novel PI3K inhibitors, which may be able to block castration-resistant PCa growth and survival [89]. In this setting, the interest in natural products has recently increased, due to their ability to specifically target the PI3K/AKT cascade. In particular, quercetin, apigenin, curcumin, genistein, sulforaphane, and EGCG have been demonstrated to attenuate PCa cell growth by downregulating this signaling pathway [90][91][92][93][94][95][96][97][98][90,91,92,93,94,95,96,97,98].
During PCa progression, both tumor invasion and chemoresistance are promoted by NF-κB. Indeed, constitutive activation of this protein has been commonly found in primary PCas and it is associated with AR loss and castration-resistant features. Thus, NF-κB is an important target for PCa management, owing to its role in tumorigenesis and therapy resistance [99]. Notably, downregulation of this protein and of its target genes has been highlighted after resveratrol, genistein, sulforaphane, ursolic acid, tocotrienol, and celastrol treatment [100][101][102][103][104][105][100,101,102,103,104,105].
Hh pathway activation is implicated in the development of different types of tumors, including PCa. In particular, many studies have pointed out that this signaling plays a crucial role in the progression of PCa to more aggressive and chemoresistant states [106]. Slusarz et al. demonstrated that seven common nutraceuticals, (i.e., genistein, curcumin, EGCG, resveratrol, apigenin, baicalein, and quercetin) can suppress the Hh pathway both in vitro and in vivo, with four of them (i.e., genistein, curcumin, resveratrol, and EGCG) decreasing not only Hh effector Gli1 expression but also Gli1 reporter activity [107].
Genome sequencing and gene expression analyses have evidenced the importance of the Wnt pathway in the development of castration resistant PCa [108]. Wnt signaling is also implicated in the cross-talk with the PCa microenvironment, where this protein is secreted by the tumor stroma and promotes therapy resistance, as well as in PCa stem cell self-renewal or expansion [109]. Preclinical studies have illustrated the potential of Wnt inhibitors in preventing PCa progression. Some of them have already been tested in phase I trials, although they have not been administered to PCa patients yet [108][109][108,109]. Interestingly, treatment of PCa cells with quercetin, curcumin, genistein, and silibinin resulted in growth suppression through Wnt cascade modulation [110][111][112][113][110,111,112,113].
MicroRNAs (miRNAs) are endogenous, ≈22 nucleotides, non-coding RNAs able to induce both transcriptional and translational arrest, thus functioning as either oncogenes or oncosuppressors, depending on the specific tumor type [114]. Concerning PCa, genistein has shown promise in modulating the levels of different oncogenic (i.e., miR221, miR222, miR151, and miR1260b) and oncosuppressor (i.e., miR-574-3p and miR34a) miRNAs, thus affecting cancer cell proliferation [115][116][117][118][119][120][115,116,117,118,119,120]. Similar encouraging data were also obtained from in vitro studies with luteolin, curcumin, resveratrol, ginsenoside Rh2, and celastrol [121][122][123][124][125][126][121,122,123,124,125,126].
