Breast cancer is a leading cause of cancer-related deaths worldwide, predominantly caused by metastasis. It is generally accepted that the pattern of breast cancer metastasis is largely determined by the interaction between the chemokine receptors on cancer cells and the chemokines expressed at the sites of metastatic disease. Chemokine receptors belong to the G-protein-coupled receptors (GPCRs) family that appear to be implicated in inflammatory diseases, tumor growth and metastasis. One of its members, C-C Chemokine receptor 7 (CCR7), binds chemokines CCL19 and CCL21, which are important for tissue homeostasis, immune surveillance and tumorigenesis. These receptors have been shown to induce the pathobiology of breast cancer due to their ability to induce cellular proliferation and migration upon the binding of the cognate chemokine receptors. The underlying signaling pathways and exact cellular interactions within this biological system are not fully understood and need further insights.
Note: The following contents are extract from your paper. The entry will be online only after author check and submit it.
1. Introduction
Since their discovery over 30 years ago, numerous chemokines have been identified, with new roles emerging past the homing of leukocytes
[1]. Chemokines or chemotactic cytokines constitute a large family of small (8–12 kDa) structurally related polypeptides that exert their functions by binding specific Gαi-protein-coupled chemokine receptors (GPCR) on the cell surface to induce chemotaxis
[2]. Structurally, to date, more than 40 human chemokines have been described. The most widely used nomenclature subdivides chemokines into four subfamilies (CC, CXC, CX3C and XC) according to the position of the first two N-terminal cysteine residues in their amino acid sequence where C represents the cysteine and X is any amino acid
[1].
Currently, 22 chemokine receptors have been identified in humans. These are G-protein-coupled receptors (GPCRs)-integral seven-transmembrane glycoproteins domains (connected by three intracellular and three extracellular loops), a short extracellular N-terminal that will bind to the ligand and an intracellular C-terminal coupled to the group of G-protein receptors. In the literature, four “atypical” receptors have been described capable of binding chemokines with high affinity with no signaling or signaling which is not mediated by G-proteins. This group of receptors has also been named “scavenger” receptors and recently renamed to “ACKR”, the acronym of atypical chemokine receptor
[3]. Receptor–ligand interaction leads to signal transduction involving G-proteins which promotes the release of intracellular second messengers such as calcium, cyclic adenosine monophosphate (cAMP) and phosphoinositides (reviewed in
[4]). Chemokines (chemotactic cytokines) are small heparin-binding proteins that are known to play a crucial role in directing the movement of cells throughout the body
[5]. Most chemokines bind several receptors and a single receptor can often bind several chemokines, forming an intertwined web in which a sole role can be played by several elements.
Chemokines and chemokine receptors are essential in dendritic cell (DC) maturation and (B and T) cells’ development
[6][7][6,7]. Indeed, recent studies indicated that chemokines have a role in the chemotaxis of particular T cell or monocyte subsets that had not been identified previously
[8][9][8,9]. The chemokine family can be either pro-inflammatory or homeostatic. The former is released due to a pathogen; an infection or other pro-inflammatory stimuli that causes the induction of chemokines that will direct the recruitment of leukocytes towards the site of injury
[10]. Depending on the type of inflammation, a different immune cell subset will be recruited to the site
[11], whilst the latter is constitutively expressed in specific tissues and has roles in tissue development (such as angiogenesis or neovascularization) or basal leukocyte migration.
A considerable body of evidence highlights the importance of chemokines and their receptors during tumor progression and metastasis. Initially, chemokines selectively regulate the recruitment and trafficking of leukocyte subsets during homeostasis and inflammation
[12][13][12,13]. However, it is becoming increasingly clear that they are also responsible for controlling the function of several tumor-promoting processes including host immune responses against malignant cells, cancer cell growth, angiogenesis and metastasis
[14][15][14,15]. Chemokine receptors are further involved in the regulation of cancer progression through the recruitment of immune cell subsets. Importantly, there is a paucity of information from in vivo experimental systems regarding the specific function of individual chemokine receptors in cancer progression, whereas only very little data exist regarding their role in primary tumor formation.
The discovery that cancer cells overexpress C-C Chemokine receptor 7 (CCR7), which directs them to organs that express their ligands CCL21 and CCL19, led to an increase in reports confirming that chemokine receptors were present in a non-random manner in many other cancers. A positive correlation between chemokine receptor expression and worse prognosis has been found in most but not all cancers. In breast cancer, it has been proved that the chemokine receptors CXCR4, CCR7, CCR6 and CXCR3 and their ligands have been associated with metastasis
[16][17][16,17]. Despite all the evidence linking CCR7 expression to a poorer prognosis, still much remains unknown about the exact mechanisms behind its upregulation and its role in breast cancer progression, which will be discussed in details in this review.
2. Chemokine Receptors in Cancer
The recruitment of immune cells to the tumor site is mediated by the actions of chemokines and their specific ligands, which have also been widely linked to cancer progression and metastasis. Upon the binding of the chemokine ligands, the receptor undergoes a conformational change. This results in the expression of several genes and activates a signaling cascade that, depending on the context, can stimulate cellular growth, migration, pseudopodia formation, adhesion, as well as angiostasis
[18].
It was thought that metastasis was regulated through factors such as the size of the vessels or the difference in pressure between the blood and the organs
[19]. However, because of the involvement of chemokine receptors in the migration of cells, they have been widely implicated in the metastasis of cancer cells throughout the body
[10]. Tumor cells, through the expression of lymphoid chemokine receptors, may exploit the physiological lymphatic trafficking system to mediate invasion into the lymphatic vasculature
[20]. Chemokine receptors can potentially facilitate tumor dissemination at numerous stages of the metastatic cascade, including vascular extravasation, protection from the host, adherence of tumor cells to endothelium, proliferation, colonization and angiogenesis
[20][21][20,21]. To date, the most recognized receptor/ligand pairs in these phenomena include CXCR4/CXCL12 and CCR7/CCL21.
3. C-C Chemokine Receptor 7 (CCR7) and Breast Cancer
The receptor CXCR4 is the most studied of all chemokine receptors in cancers. Its roles in breast cancer, including cell survival, proliferation, motility, invasion, angiogenesis, recruitment and activation of a number of different cell types, as well as metastasis are well documented
[22][80]. CCR7 is often up-regulated together with CXCR4 in cancer
[23][81]. In addition, these two receptors form a heterodimer on metastatic breast cancer cells, which activates alternative signaling pathways and promotes a metastatic phenotype
[24][82].
In breast cancer, hypoxia has been shown to increase CCR7 expression through the hypoxia-inducible factor 1 (HIF-1)-mediated activation of the endothelin receptor A
[25][83]. Epigenetic factors could also play a role in CCR7 upregulation, with histone deacetylation and DNA methylation playing a role in gene activation
[26][84]. Despite all the evidence linking CCR7 expression to a poorer prognosis, still much remains unknown about the mechanisms behind its upregulation.
The interaction between CCR7 and CCL21 has been shown to improve the immunogenicity of CCR7-expressing breast cancer cells
[27][85]. It also induces actin polymerization and pseudopodia formation, resulting in increased cell motility as illustrated in ()
[28][86].
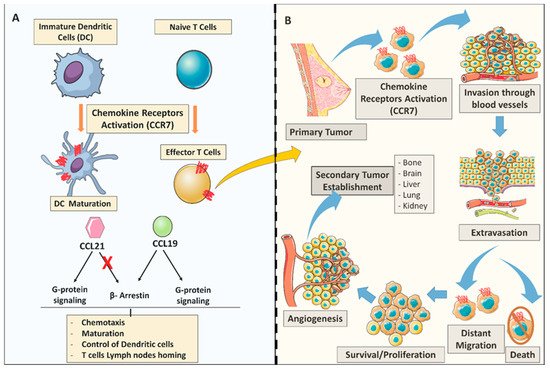
Figure 1. General C-C Chemokine receptor 7 (CCR7) chemokine dual role in the immune system and breast tumor metastasis. (A) CCR7 role in the adaptive immune system; CCL19/CCL21 chemokine expression activates CCR7 to promote dendritic cells (DC) and T-cell maturation. CCL21 and CCL19 chemokines determine differential signaling. Both chemokines CCL21 and CCL19 have the ability to display G-protein signaling cascades, however only CCL19 can trigger the β-arrestin recruitment. (B) Chemokine receptors in the metastatic process. (As illustrated) CCR7 chemokine receptor activation may play key roles in several steps during the process of metastasis; arrest; dissemination; invasion, extravasation; survival/proliferation) all under the control of the immune system trafficking to the site of inflammation.
3.1. Molecular Aspects of C-C Chemokine Receptor 7 (CCR7) Signaling Cascades in Breast Cancer
Chemokine signaling in which a cell responds to a self-secreted chemokine is known as autocrine signaling, as opposed to paracrine signaling in which a cell responds to a chemokine secreted by a neighboring cell
[29][87]. Both autocrine and paracrine signaling are well established mechanisms that drive tumor cell phenotype and migration.
There are a large number of chemokines that regulate the downstream effector molecules which may account for the various effects of these recipients in tumor pathology. To date, the effector molecules and unique mechanisms controlling cell chemotaxis, proliferation, survival and motility have been highly considered in chemokine receptor signaling in cancer
[30][31][88,89].
Measuring chemokine ligands’ ability to induce early physiological changes, chemotaxis, actin polymerization and calcium mobilization is routinely performed in vitro to assess chemokine receptor functionality
[32][90]. These findings provide evidence that CCR7 activation is cell-dependent via distinct mechanisms of actions. A number of key metastasis steps have been shown to facilitate tumor dissemination, including the endothelium adhesion of tumor cells, blood vessel extravasation, protection from host responses, angiogenesis, proliferation and metastatic colonization
[33][91].
It is thought that chemokine responses are activated through key survival pathways such as the PI-3K and AKT, JAK/STAT, ERK 1/2, JNK, GSK-3α/β and MAP kinase
[34][35][36][73,92,93]. Moreover, the exact signaling mediators in primary and invasive tumors are yet to be elucidated, although chemokine signals have been characterized in leukocytes with few currently available evidences on other cell types. The CCL21 binding to CCR7 has been reported to stimulate the activation phosphorylation of AKT (PI-3K pathway) that mediates a wide variety of intracellular targets to induce cell survival and inhibit apoptosis in many different types of cancer cells
[37][38][94,95], as well as an implied effect on cell proliferation and motility
[39][96]. This was further confirmed by the ability of CCR7 ligands to induce early migratory responses such as cytoskeleton remodeling and chemotaxis in metastatic breast cancer cells
[40][97]. CCR5 and CCR7 enhance protooncogene c-Fos expression by JAK/STAT pathway in leukemia cells
[41][98]. The activation of ERK in lymphoma and non-small lung cancer cells is also implicated in CCR3, CCR7 and CCR8 by inducing phosphorylation, proapoptotic protein inactivation and the activation of survival mechanisms
[42][43][47,99].
Chemotactic responses and actin polymerization are not induced following CCR7 ligand-binding activation in non-metastatic breast cancer cells. Chemokine signaling pathways are mediated by the mobilization of the calcium flux through the activation of GPCRs, specifically the Gαi subunit that results from the inhibiting of adenylyl cyclase-mediated cAMP
[44][45][100,101]. The pre-treatment with the Gαi inhibitor “pertussis toxin” impairs this calcium induction responsiveness. In addition, Adenylyl cyclase-mediated cAMP, stimulated by forskolin, is inhibited by CCL19 ligand treatment only in invasive breast cancer cells
[46][102]. Therefore, functional Gαi signaling after chemokine treatment was only observed in the metastatic breast cancer cells. However, even after the addition of a phosphatidic acid, non-chemokine GPCR and non-metastatic cells did not respond. This might have been caused by the disruption of the upstream signaling events.
Hematopoietic and non-hematopoietic tumors metastatic to lymph nodes are correlated with tumor-dependent CCR7 expression. Physiologically, this indicates mature DCs and naive T lymphocytes recruitment to lymph nodes
[34][47][73,103]. However, the induced expression of CCR7 in breast cancer cells switches metastasis from the lung to lymph nodes, indicating that the organ specificities of metastatic cancer cells might be sufficient by a single chemical receptor
[48][31]. In some experimental settings, epithelial-to-mesenchymal transition (EMT) was triggered by a PI3K-mediated activation of snail and glycogen synthase kinase (GSk)-3β in hepatocellular carcinoma cells through CCL21/CCR7 and CXCL5/CXCR2 binding
[49][104]. Moreover, TGF-β is reported to stimulate EMT through crosstalk with NF-κB signaling pathway in gastric cancer cells
[50][51].
The pathways used by CCR7 to facilitate metastasis are not fully understood. It has been reported that CCR7 facilitates cancer progression by manipulating cancer cells’ anoikis and mobility
[24][82] as well as controlling the tumor microenvironment, including the inflammatory responses, immune tolerance and T cell activation
[51][52][105,106]. Downstream potential signaling mediators of CCR7 have been reported to be associated with metastasis, such as ALDH3A2, AnXA6, EpCAM, and PVR
[53][107]. MMPs play an important role in tumor cell invasion and migration. It has been illustrated that CCR7 triggers AKT which eventually leads to the secretion of MMP-2/9. The silencing of CCR7 thus acts as a targeted therapy to inhibit AKT and MMPs expression and attenuates MCF-7 cell proliferation, invasion and EMT
[54][45]. A schematic illustration of CCR7 binding to the cognate ligands CCL19/CCL21 to induce several signaling transduction pathways in breast cancer is shown in ().
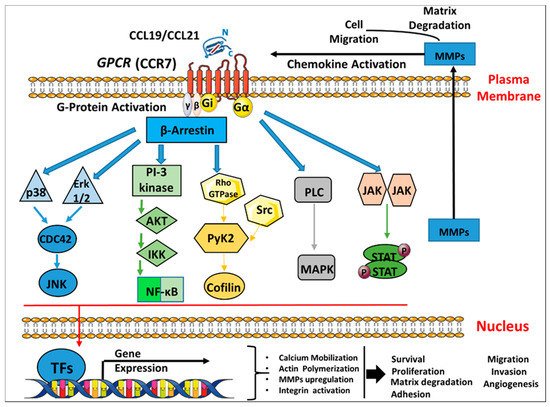
Figure 2. CCR7 chemokine receptor binding to the cognate ligands CCL19/CCL21 inducing signaling transduction pathways in breast cancer. Schematic representation of the seven transmembrane G-protein coupled receptor (CCR7) activation and internalization by ligand binding which triggers several signaling pathways cascades as follows: a. The binding of chemokine ligands CCL19/CCL21 to their CCR7 GPCRs leads to the activation of a G-α subunit and a Gi-βγ heterodimer. b. This initiates downstream signaling effectors that further lead to signaling cascades, such as the activation of ERK1/2, PI3K/AKT, Rho GTPases, MAPK, and JAK/STAT, which can promote the transcription and expression of different genes such as MMPs. c. All the above induce broad biological effects including chemotaxis, cell survival/proliferation, matrix degradation, reorganization of the actin cytoskeleton, adhesion, cell migration, invasion and angiogenesis. GPCR, G-protein-coupled receptors; ERK, extracellular signal-regulated kinase; JNK: c-Jun N-terminal kinase; PI3K, Phosphatidyl inositide-3-kinase; AKT, protein kinase B; NF-κB, nuclear factor-κB; Rho, Ras homolog gene family; PLC, phospholipase C; MAPK, mitogen-activated proteins kinase; JAK, Janus-activated kinase; TFs: Transcription factors, MMP, matrix metalloproteinase.
CCR7 expression is regulated by transcription factors, epigenetic and miRNAs mechanisms. Transcription factors such as SP-1, AP-1, NF-κB and NF-AT1 can bind and regulate CCR7’ expression
[55][56][108,109]. Nevertheless, it has not been well described whether these factors are correlated with tumor metastasis. The constituent expression of Ets-1, an oncogene that is involved in EMT, is well associated with the expression of CCR7 and cell migration activity in MDA-MB-231
[57][58][110,111].
Studies in the field of breast cancer have shown that the correlation between the expression of CCR7 and clinical pathogenicity in human breast cancer is important, including vascular invasion, lymph node metastasis and tumor grade
[59][112]. However, other reports found contradictory results
[60][113]. Hence, to gain greater certainty, more studies are recommended to determine the association between patient survival, recurrence risk and metastasis with the expression of CCR7. Moreover, CCR7 expression may enhance breast cancer cells’ migration to lymph nodes. Nevertheless, it is still not fully understood how this migration has contributed to a better prognosis. One explanation could be that positive CCR7 cancer cells primarily target metastasis to lymph nodes as well as empower the immune system to eliminate escaped cancerous cells
[61][114]. Remarkably, Gracio et al. reported that the alternative splicing of CCR7 has been correlated to clinical results
[62][115]. Therefore, the interactions between this essential post-transcriptional regulation and patient survival should be carefully investigated in correlation with CCR7 alternative splicing in breast cancer
[63][116]. Nevertheless, this issue should be addressed in future studies to reveal the impact of CCR7 expression.
3.2. The Expression and Functional Role of C-C Chemokine Receptor 7 (CCR7) in Breast Cancer Cells in Vitro
The up-regulation of specific chemokine/receptor pairs has been reported in many human cancers including lung, breast, colon, prostate, gastric and melanoma
[33][64][91,117], although at this point their precise mechanisms of action at each recurring event of metastasis are still being investigated. Recently, the expression of CXCR4 and CCR7 have received considerable attention in tumor cells for their direct involvement in metastasis. A variety of cancer cell lines and primary tumors tissues reported an expression of these chemical receptors and the interaction with the associated ligands to promote
the in vitro and in vivo movement of cancer cells
[65][38].
The CCR7 expression was evaluated in breast cancer cells in vitro to ascertain whether the rates of expression of a chemokine receptor contribute to the pathogenicity of breast cancer. A group of cell lines was used; each was originally derived from patients with different types of metastatic breast disease displaying various degrees of invasiveness. The examination of the CCR7 expression revealed significant levels of expression in different breast cell lines. Studies reported low CCR7 mRNA levels in MCF-7, MDA-MB-231 and T47D
[18][66][18,118], no expression at mRNA levels in MDA-MB-231
[67][119], and inducible levels in MCF-7, MDA-MB-231, and SKBR3
[25][83]. Clinical studies revealed that breast cancer tumors show cytoplasmic CCR7 expression
[68][69][120,121]. The same cytoplasmic-confined phenomena were also reported later in 4T1 cells
[70][122]. These data confirm the internalization and activation of the CCR7 receptor upon ligand binding. It is also worth mentioning that CCR7 expression was detected with similar levels in both immortalized normal breast epithelial cells (MCF10A) as well as in a highly invasive breast cancer cell line (MDA-MB-231)
[52][106]. These data strongly suggest that CCR7 expressions are not strictly limited to invasive cells and would not be a useful predictor of metastasis in aggressive breast cancer. Although the CCR7 physiological function is known in normal breast epithelial cells, their exact role remains unknown but valuable to be determined in non-metastatic breast cancer cells. Moreover, it can be predicted that cells gain selective advantages to proliferate, colonize, survive and migrate at secondary sites when chemokine receptors are activated through cancer progression.
3.3. The Role of C-C Chemokine Receptor 7 (CCR7) on Breast Cancer Metastasis in Vivo
Metastasis is one of the problematic tumor processes to study in vitro since the tumor progression relies on sequential events that are dependent on the characteristics of the tumor cells and their interactions with the tissue environment
[71][123]. Therefore, more relevant studies of metastasis are thought to originate from those performed in vivo. Significantly, mice models of different human cancers have become a central part of many types of biomedical research as they provide the most experimentally accessible mammalian model that shares genes, physiology and organ structure with the human system
[72][124].
To provide further insight into chemokine-mediated metastatic mechanisms, the expression of CCR7 on mammary epithelial cell lines was modulated using an shRNA system based on the ability to deliver and stably express shRNAs, to study the effect of chemokine modulation on the metastatic propensity of breast cancer cells in vivo
[73][125]. The silencing of CCR7 decreased the in vitro proliferation, migration and invasion of CCL21/CCL19-induced MCF-7 and MDA-MB-231 breast cancer cells
[54][74][45,126]. Furthermore, the depletion of CCR7 in BALB/c mice inhibited orthotopically injected 4T1 cells metastasis, while tail veins’ injection had no impact on breast cancer metastasis. These outcomes were consistent with the previous findings, showing that CCR7 did not affect liver and lung metastasis when pancreatic cancer cells (TD-2) were injected via tail veins, but rather increased B16 cell metastasis to lymph nodes
[75][127]. On the other hand, CCR7 overexpression in MMTV-PyMT-mediated breast cancer cells, injected orthotopically into FVB mice, significantly increased lymph node metastasis
[48][31].
CCR7 expression has been reported in particular to increase B16 metastatic capacity via the lymphatic system but not through a blood-borne pathway
[76][128]. At first, CCR7 had a significant role as stimulated DCs migrated through various lymph vessels to specific lymph nodes. Subsequently, it was suggested that neoplastic cells also enter lymph vessels to improve lymphatic dissemination, shown for B16 melanoma cells
[77][129]. The formation of the CCR7 metastatic lymph node expressing B16 cells was effectively blocked by the neutralization of anti-CCL21
[77][129], which confirmed the role of CCR7 during metastasis. Given the contrasting studies, the exact relationship between the expression of chemokine in metastatic tumor progression and their potential role still requires considerable attention.
Higher levels of CCR7 mRNA was detected in cancer tissue compared to the normal counterpart lung tissues, suggesting that CCR7 expression could be used to predict the metastasis of the lymph node before surgery as a diagnostic tool to improve the overall treatment plan for non-small cell lung cancer (NSCLC)
[78][55]. Moreover, the CCR7 presence was directly related to the lymph node metastases development in NSCLC
[79][35]. In addition, the expression of chemokine receptors in clinical samples may help correlate their levels with the outcome of cancer patients. Interestingly, based on the expression status of hormonal receptors, triple-negative breast cancer (TNBC) is associated with poor prognosis, higher metastasis and worse outcomes than other subtypes due to the absence of efficient targeted therapies
[80][130]. In breast cancer tissues, CCR7 has been highly expressed especially in the luminal B-type and TNBC compared to the luminal A type
[61][81][114,131]. Therefore, tumor microenvironment signals are likely to be interpreted differently in luminal-B cells than those in luminal-A cells. Thus, the tumor microenvironment factors do not inhibit the responsiveness of luminal-B cells to chemotactic cues mediated through the CCR7/CCL21 axis
[82][46].
Several experimental approaches using specific chemokine antagonists, neutralizing antibodies, knockout mice and targeted gene disruptions have been reported to be useful tools for discerning the role of chemokines and their receptors in vivo
[83][84][132,133]. Therefore, these methods may potentially provide a basis for the development of future therapeutic agents. Studies involving the inhibition of CCR7 expression on cancer cells are yet to be explored at both the in vitro and in vivo level.