Bone morphogenetic protein-7 is (BMP-7) is a potent anti-inflammatory growth factor belonging to the Transforming Growth Factor Beta (TGF-β) superfamily. It plays an important role in various biological processes, including embryogenesis, hematopoiesis, neurogenesis and skeletal morphogenesis. BMP-7 stimulates the target cells by binding to specific membrane-bound receptor BMPR 2 and transduces signals through mothers against decapentaplegic (Smads) and mitogen activated protein kinase (MAPK) pathways. To date, rhBMP-7 has been used clinically to induce the differentiation of mesenchymal stem cells bordering the bone fracture site into chondrocytes, osteoclasts, the formation of new bone via calcium deposition and to stimulate the repair of bone fracture. However, its use in cardiovascular diseases, such as atherosclerosis, myocardial infarction, and diabetic cardiomyopathy is currently being explored. More importantly, these cardiovascular diseases are associated with inflammation and infiltrated monocytes where BMP-7 has been demonstrated to be a key player in the differentiation of pro-inflammatory monocytes, or M1 macrophages, into anti-inflammatory M2 macrophages, which reduces developed cardiac dysfunction.
- atherosclerosis
- myocardial infarction
- diabetic cardiomyopathy
- inflammation
1. Introduction
| Types | Alternate Names | Tissues that Express | Functions | Receptors |
|---|---|---|---|---|
| BMP-1 | BMP-1 is a metalloproteinase | major end organs (heart, lung, liver, pancreas, kidney, and brain), lymphoid organs (bone marrow, thymus, spleen and lymph nodes), exocrine glands (prostate and mammary gland) organ protectors (muscle and bone) | Metalloprotease that cleaves COOH–propeptides of procollagens I, II, and III/induces cartilage formation/cleaves BMP antagonist chordin |
_____ |
| BMP-2 | BMP-2A, XBMP2, xBMP-2, MGC114605 |
major end organs (lung, pancreas, and kidney), lymphoid organ (spleen) | Induces bone and cartilage formation. Plays a role in skeletal repair and regeneration/heart formation | ALK-2, 3, 6 BMPR-II; ActR-IIA, ActR-IIB |
| BMP-3a & 3b |
Osteogenin, BMP-3A |
major end organs (brain, heart, pancreas), exocrine gland (prostate), organ protector (skeletal muscle), lymphoid organs (bone marrow, spleen and thymus), BMP-3b also expresses in spinal cord |
Negative regulator of bone morphogenesis Cell differentiation regulation; skeletal morphogenesis; Regulates cell growth and differentiation in both embryonic and adult tissues |
ALK-4 ActR-IIA, ActR-IIB |
| BMP-4 | BMP-2B, BMP2B1, ZYME, OFC11, MCOPS6 |
major end organs (brain, heart, pancreas, liver, lung, kidney), exocrine gland (prostate), organ protector (skeletal muscle), lymphoid organs (bone marrow, spleen and thymus), spinal cord | Skeletal repair and regeneration; kidney formation; Induces cartilage and bone formation; limb formation; tooth development. | ALK-2,3,5,6 BMPR-II, ActR-IIA |
| BMP-5 | MGC34244 | major end organs (brain, heart, pancreas, liver, lung, kidney), exocrine gland (prostate), organ protector (skeletal muscle), lymphoid organs (bone marrow, spleen and thymus), spinal cord | Limb development; induces bone and cartilage morphogenesis; connecting soft tissues | ALK-3 BMPR-II; ActR-IIA, ActR-IIB |
| BMP-6 | Vgr1, DVR-6 | major end organs (brain, heart, pancreas, liver, lung, kidney); exocrine gland (prostate); organ protector (muscle and bone), lymphoid organs (bone marrow, spleen and thymus); spinal cord |
Cartilage hypertrophy; bone morphogenesis; nervous system development; Plays a role in early development | ALK-2, 3, 6 BMPR-II; ActR-IIA, ActR-IIB |
| BMP-7 | OP-1 | major end organs (brain, heart, pancreas, liver, lung, kidney), exocrine gland (prostate) organ protector (skeletal muscle), lymphoid organs (bone marrow, spleen and thymus), spinal cord. | Skeletal repair and regeneration; kidney and eye formation; nervous system development plays a major role in calcium regulation and bone homeostasis |
ALK 2, 3, 6 BMPR-II; |
| BMP-8a & 8b |
OP-2, FLJ14351, FLJ45264 OP-3, PC-8, MGC131757 |
major end organs (brain, heart, kidney, lung, liver, pancreas), exocrine gland (prostate), organ protector (skeletal muscle), lymphoid organs (spleen, thymus bone marrow) spinal cord | Induces cartilage formation; Bone morphogenesis and spermatogenesis; calcium regulation and bone homeostasis. | ALK 2; 3; 4; 6; 7 BMPR-II; ALK3,6 BMPR-II; ActR-IIA, ActR-IIB |
| BMP-9 | GDF-2 | major end organ (liver) | Bone morphogenesis; cholinergic neurons development; in glucose metabolism; potent inhibitor of angiogenesis |
ALK-1,2 BMPR-II; ActR-IIA, ActR-IIB |
| BMP-10 | MGC126783 | major end organs (brain, heart, kidney, lung, liver, pancreas), exocrine gland (prostate), organ protector (skeletal muscle), lymphoid organs (spleen, thymus, bone marrow) spinal cord. | Heart morphogenesis maintains the proliferative activity of embryonic cardiomyocytes by preventing premature activation of the negative cell cycle regulator; inhibits endothelial cell migration and growth |
ALK-1, 3, 6 ActR-IIA, ActR-IIB |
| BMP-11 | GDF-11 | major end organs (brain, pancreas), exocrine gland (prostate), lymphoid organs (spleen, thymus bone marrow) spinal cord. | Pattering mesodermal and neural tissues, dentin formation | ALK-3, 4, 5, 7 BMPR-II; ActR-IIA, ActR-IIB |
| BMP-12 | GDF-7, CDMP-3 | _____ | Ligament and tendon development/sensory neuron development | ALK-3, 6 BMPR-II; ActR-IIA |
| BMP-13 | GDF-6, CDMP-2, KFS, KFSL, SGM1, MGC158100, MGC158101 |
_____ | Normal formation of bones and joins; skeletal morphogenesis and chondrogenesis Plays a key role in establishing boundaries between skeletal elements during development |
ALK-3, 6 BMPR-II; ActR-IIA, ActR-IIB |
| BMP-14 | GDF-5, CDMP-1, OS5, LAP4, SYNS2, MP52 |
sensory organs (eye, skin), major end organs (brain, heart; kidney, liver, lung), embryonic tissue, mixed connective tissue, pituitary gland, salivary gland; exocrine gland (prostate), reproductive system related (uterus), lymphoid organ (bone marrow) | Bone and cartilage formation; Skeletal repair and regeneration |
ALK-3, 6 BMPR-II; ActR-IIA |
| BMP-15 | GDF-9B, ODG2, POF4 | _______ | Oocyte and follicular development | ALK-6 |
| BMP-16 | _____ | embryonic tissue; reproductive system (testis) |
Skeletal repair and regeneration Essential for mesoderm formation and axial patterning during embryonic development |
_____ |
| BMP-17 | _____ | major end organ (brain, lung, liver, pancreas, spleen) lymphoid organ (lymph node); exocrine gland (mammary gland); sensory organ (skin); reproductive organ (testis); bladder; embryonic tissue; intestine; joints; | Required for left-right axis determination as a regulator of LEFTY2 and NODAL | _____ |
| BMP-18 | _____ | major end organ (brain), embryonic tissue, reproductive system (testis) |
Required for left-right (L-R) asymmetry determination of organ systems in mammals. May play a role in endometrial bleeding | _____ |
2. Structure of BMP-7
BMP-7 is expressed by several tissues, including, sensory organs (eye and skin), major end organs (heart, lung, liver, pancreas, kidney, and brain), lymphoid organs (bone marrow, thymus and lymph nodes), the reproductive system (testis, ovary, uterus and placenta), exocrine glands (prostate and mammary gland), and organ protectors (muscle and bone) [22][43][44][45][46][47][48][49][22,43,44,45,46,47,48,49]. It is synthesized in the cells as pro-protein form of 431 amino acid residues, including N-terminal signal peptide of 29 amino acid residues, a pro-peptide of 263 amino acids, and a mature peptide of 139 amino acid residues [50] (Figure 1). During processing, pro-BMP-7 is hydrolyzed in the cell by furin-like proteinase on its carboxy terminal, where it is converted into mature BMP-7 of 139 amino acid residues and secreted into the extracellular matrix [51]. BMP-7 is approximately a 35 kDa glycoprotein with three N-glycosylation sites and seven cysteine residues involved in three intramolecular disulfide bonds Cys38-104, Cys67-136 and Cys71-138 [52]. More importantly the intermolecular disulfide bond formed via the 103rd cysteine form dimers in two mature BMP-7 monomers with enhanced biological activity. BMP-7 has the ability to form homodimers as well as heterodimers to induce bone formation. It has been reported that BMP-7 can form heterogenous dimers with other BMPs, specifically, BMP-2 and BMP-4 [53][54][55][53,54,55]. However, heterodimers are more potent than homodimers in osteogenic differentiation assays [56][57][58][56,57,58]. Moreover, it has been demonstrated that the biological activity of these heterogenous dimers is almost 20 times higher than that of homodimers [39][58][59][39,58,59]. These heterodimers also showed enhanced activity in embryonic assays of Xenopus and Zebrafish [60][61][60,61]. According to these studies, co-injection of RNA encoding BMP-7 with BMP-2 or BMP-4 into embryonic blastomere enhanced embryo ventralization and patterning compared with individual injection. Additionally, combined injection of purified recombinant proteins of BMP4/7 or BMP2/7 increased BMP signaling (SMAD pathway) in Xenopus and Zebrafish, whereas varied concentrations of individual injections of homodimers did not have that level of BMP signaling alterations, suggesting that heterodimes are more potent in BMP cell signaling [55][61][62][55,61,62].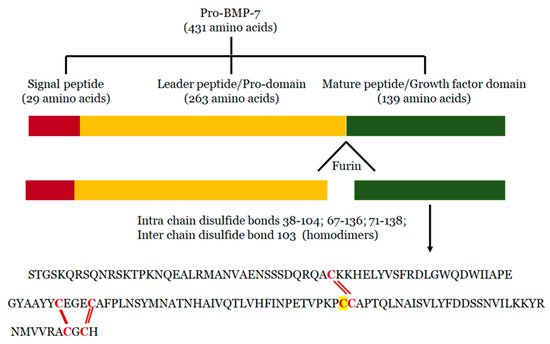
3. Mechanisms of BMP-7
BMP-7 binds to bone morphogenetic protein receptor 2 (BMPR2) on the surface of cells and activates two major signaling pathways: 1) Canonical/Smad dependent and 2) Non-canonical/Smad independent pathway [65][66][65,66] (Figure 2).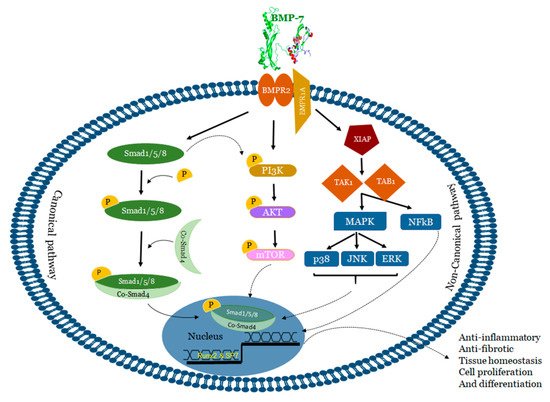
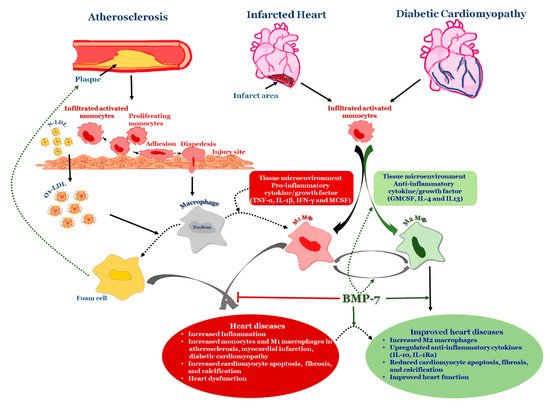
4. BMP-7 as an Anti-Inflammatory Agent in Atherosclerosis
Atherosclerosis is a serious cardiovascular condition that involves the constriction of the arterial wall leading to the development of myocardial infarction. Atherogenesis is regulated by cholesteryl ester (CE) accumulation, foam cell formation, smooth muscle cell migration, necrotic core formation, and increased calcification [66][99][100][101][66,130,131,132]. Moreover, the developed atherogenesis creates turbulence in blood flow leading to plaque rupture and thrombosis. Although these atherogenic factors are well-established, recent data suggests the involvement of modified LDL, extracellular components in the plaque activation and rupture [102][133]. Therefore, atherosclerosis was considered to be the product of lipoprotein accumulation, particularly LDL in the arterial wall [103][104][134,135]. Recently, it is speculated that atherosclerosis is a complex process that involves the participation of both immune systems, oxidative stress, various cell types, receptors, lipids, enzymes, signaling pathways, trace elements, and other products [105][106][107][136,137,138]. Inflammation and oxidative stress are considered to be major players in the progression of the disease [108][109][110][111][139,140,141,142]. Altered vessel wall structure and disturbed blood flow patterns include inflammation and varied stress levels in developed atherosclerosis [112][143]. Despite the abundance of research literature on the topic, the role of lipids, especially fatty acids and their oxidation products like peroxidized linoleic acid (HPODE), 4-hydroxynonenal (HNE), oxo-nonanoic acid (ONA), and their interaction with inflammatory molecules such as oxidized LDL, phospholipids, TNF-α, vascular cell adhesion molecule (VCAM1) in many of these processes are poorly understood. Monocytes, which are precursors of macrophages as well as dendritic cells (DCs) and migrate into the areas of “injury” as a result of a chemotactic stimuli such as monocyte chemotactic protein 1 and 3 (MCP-1&3). Migration of monocytes into the arterial wall has been considered as one of the initial events in atherogenesis which persists in different stages of disease progression [109][110][111][140,141,142]. In tissues, based on the environmental growth factors and pro-inflammatory cytokines, monocytes differentiate into either M1 macrophages or DCs. Monocyte adherence, their differentiation into pro-inflammatory macrophages/dendritic cells that release pro-inflammatory cytokines which are involved in the generation of complex pathophysiology of atherosclerosis [109][140] (Figure 3). Macrophages were initially viewed as a mere scavenger of altered lipoproteins. However, the presence of macrophages along with lymphocytes in atherosclerotic plaques showed enhanced inflammatory immune response and release of pro-inflammatory molecules. The specific roles of different stages of atherosclerosis and presence of these inflammatory macrophages, foam cells, lymphocytes, and vascular smooth muscle cells are not yet completely understood. For example, M2 macrophages, are known for high endocytic clearance capacity due to their higher expression of scavenger receptors (SR) during wound healing and repair processes [113][144]. Van Tits et al. demonstrated that M2 macrophages are susceptible in forming foam cells in presence of oxidized LDL and shift towards the M1 phenotype with enhanced secretion of the pro-inflammatory cytokines IL-6, IL-8 and MCP-1 [114][145]. Furthermore, this increased production of pro-inflammatory cytokines by polarized M1 macrophages from M2 macrophages which are residing in subendothelial space of the vessel wall might lead to the initiation of the inflammatory cascade that mediates disease progression [114][145]. Similarly, in human atherosclerotic lesions different macrophage phenotypes exist in different plaque locations. M2 (CD68+ CD206+) macrophages were located in plaque stable zones far from the lipid core, whereas M1 (CD68+ CCL2+) macrophages exhibited a distinct tissue localization pattern [115][146] suggesting that the tissue microenvironment decides the fate of macrophage polarization. Subsequent research studies confirmed this finding by demonstrating the presence of lipid droplets in CD68+ CD206+ macrophages in comparison with CD68+ CD206− macrophages [116][147]. This discovery suggests that despite the anti-inflammatory nature of M2 macrophages they tend to form foam cells, a significant contributor of atherogenesis.