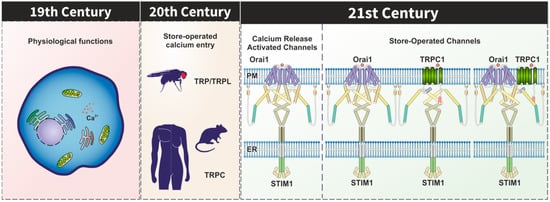
Transient receptor potential (TRP) proteins form non-selective Ca2+ permeable channels that contribute to the modulation of a number of physiological functions in a variety of cell types. Since the identification of TRP proteins in Drosophila, it is well known that these channels are activated by stimuli that induce PIP2 hydrolysis. The canonical TRP (TRPC) channels have long been suggested to be constituents of the store-operated Ca2+ (SOC) channels; however, none of the TRPC channels generate Ca2+ currents that resemble ICRAC. STIM1 and Orai1 have been identified as the components of the Ca2+ release-activated Ca2+ (CRAC) channels and there is a body of evidence supporting that STIM1 is able to gate Orai1 and TRPC1 in order to mediate non-selective cation currents named ISOC. STIM1 has been found to interact to and activate Orai1 and TRPC1 by different mechanisms and the involvement of TRPC1 in store-operated Ca2+ entry requires both STIM1 and Orai1. In addition to the participation of TRPC1 in the ISOC currents, TRPC1 and other TRPC proteins might play a relevant role modulating Orai1 channel function.
- TRPC1
- STIM1
- Orai1
- calcium influx
- store-operated Ca2+ entry (SOCE)
1. Introduction
| Orai1 Channels | Ora1-TRPC Channels | References | |
|---|---|---|---|
| Current Voltage (I–V) profile | Inwardly rectifying | Inwardly rectifying | [5][6][7][20,21,22] |
| Positive reversal potential ~ + 50 mV | Positive reversal potential 0 to ~ + 10 mV |
||
| Permeability and Selectivity | Ca2+ | K+, Na+, Cs+, Ca2+ and Ba2+ | [4][8][4,23] |
| Low to Cs3+ | |||
| Conduct Na+, Li+ and K+ in DVF solutions | |||
| Activation | Store depletion via STIM1 SOAR region | Store depletion via STIM1 SOAR and polibasic C-terminus regions | [9][10][24,25] |
| Endogenous current size | 0.1–0.2 pA/pF at −100 mV | [11][26] | |
| Fast Inactivation | Ca2+ | n/d | [12][13][27,28] |
| STIM1 CMD | |||
| Orai1 68–91 aa | |||
| Orai1 137–173 aa | |||
| Slow inactivation | Mitochondria | n/d | [14][15][16][29,30,31] |
| STIM1 390–391 aa | |||
| SARAF | |||
| Inhibitors | 2-APB (30–50 µM) | n/d | [17][18][19[22][32,33][,3420][,3521],36,37] |
| La3+ and Gd3+ (100 µM) | |||
| Low pH = 6.7 | |||
| Synta 66 | |||
| GSK-7975A GSK-5503A |
|||
| AnCOA4 (~5 µM) |
2. TRPC Channels in the STIM1–Orai1 Scenario
A new scenario emerged in the study of SOCE after the identification of Orai1 and Stim1 as the key components of the CRAC (Ca2+ release-activated Ca2+ channels). STIM1 was identified as the Ca2+ sensor in the ER which communicates the Ca2+ content of the stores to the channels in the plasma membrane, while Orai1 was identified as the pore subunit of the CRAC channel in the plasma membrane [38][39][40][41][38,39,40,41]. The expression of splice variants of STIM1 and Orai1 with functional and biophysical differences have been demonstrated in mammalian cells. STIM1L, a longer splice variant of STIM1 described in adult human muscle fibers, displays a fast full SOCE activation compared to STIM1 [42]. Regarding to Orai1, two different variants generated by alternative translation initiation, Orai1α and Orai1β, have been shown to drive ICRAC and ISOC currents [43][44][43,44]. In addition to these variants, mammalian cells also express other STIM and Orai isoforms involved in the generation of ICRAC currents. STIM2 is a more sensitive ER Ca2+ sensor than STIM1, but it promotes a weaker CRAC channel activation [45]. Three variants of STIM2, (STIM 2.1, STIM2.2, and STIM2.3) with different roles in the modulation of SOCE have been identified. While STIM2.1 has been described to play an inhibitory role, STIM2.2 has been shown as an activator of SOCE. The function of the STIM2.3 variant still remains unclear [46][47][46,47]. Orai2 and Orai3 proteins have also been shown to drive ICRAC currents after depletion of the intracellular stores [48][49][50][48,49,50] and their regulation and physiological role are less known as compared to Orai1. Therefore, it is currently widely established that the Orai-STIM complex, mainly Orai1-STIM1, constitutes the highly selective CRAC channel. TRPC1 was the first candidate proposed as SOC channel in Chinese hamster ovary cells [51] and monkey COS cells [52] by the expression of TRPC1A, a splice variant of TRPC1, and the expression of a full-length cDNA encoding human TRPC1, respectively. In both cases, the consequence was an increased SOCE after depletion of the intracellular Ca2+ stores. Later, the role of TRPC1 as the SOC channel was confirmed using different approaches in a large number of human cells, including submandibular gland cells [53], endothelial cells [54] and platelets [55], among others. However, the involvement of TRPC channels in SOCE has long been controversial with different studies providing evidence against a functional role of TRPC channels in SOCE. For instance, overexpression of TRPC channels, including TRPC3 [56][57][56,57], has been found to induce non-capacitative Ca2+ entry downstream of phospholipase C in a variety of cell models. A major problem for the involvement of TRPC channels in SOCE is that these channels cannot reproduce the biophysical properties of ICRAC. Nevertheless, as ICRAC is not the only store-operated Ca2+ current, this observation does not rule out the possibility that TRPC channels also participate in SOCE under certain scenarios, such as the assembly with the STIM1-Orai1 complex. In the new STIM1-Orai1 scenario for SOCE, it was soon reported that both proteins together with TRPC1 are assembled to form a dynamic STIM1-Orai1-TRPC1 ternary complex that drives the ISOC current [7][58][59][60][22,58,59,60]. Upon store depletion, STIM1 activation promotes its oligomerization and translocation to the ER-PM junctions where it binds Orai1 [58][59][58,59] and TRPC1 [59][61][62][59,61,62] in lipid rafts domains, gating both Ca2+channels [63][64][63,64]. STIM1 mediates Orai1 activation by the interaction of the cytosolic STIM1-Orai1 activation region (SOAR) of STIM1 [9][24] with two STIM1-bindings sites located at the C- and N-termini of Orai1 [65][66][67][65,66,67]. The SOAR region is also required for STIM1-TRPC1 interaction; however, it is not sufficient to activate TRPC1 [9][24]. The activation of TRPC1 requires electrostatic interaction between highly positively charged lysines (684KK685) located in polybasic lysine-rich domain (K-domain) of the STIM1 C-terminus with the conserved, negatively charged, aspartate residues in TRPC1 (639DD640) and equivalent residues in other TRPC channels [10][25]. However, there is no evidence about the domains of Orai1 and TRPC1 involved in their interaction, suggesting that TRPC1-Orai1 binding could be indirectly mediated by STIM1 or still unidentified adaptor proteins [68][69][68,69]. The first evidence of the dynamic assembly of the STIM1-Orai1-TRPC1 ternary complex was found using immunofluorescence and confocal microscopy assay in human salivary gland cells. In resting conditions, STIM1 shows a diffused cytosolic localization while TRPC1 is located in the PM colocalizing with Orai1, although it is also expressed in the cytosolic region. After Ca2+ store depletion, STIM1 co-localized in the PM with both proteins, TRPC1 and Orai1, without modifying the TRPC1 and Orai1 colocalization [59]. Different studies have demonstrated that a functional Orai1 plays an essential role in the STIM1-Orai1-TRPC1 complex formation using different approaches. In human platelets, the STIM1-Orai1-TRPC1 ternary complex formation, including Orai1-STIM1 binding, was demonstrated using immunoprecipitation assays and the electrotransjection with an anti-Orai1 C-terminal antibody impairs the interaction between STIM1 and TRPC1, as well as SOCE activation after intracellular Ca2+ store depletion [58]. In Orai1 knockdown HEK-293 by siRNA-mediated gene silencing, cell transfection with the dominant negative mutants Orai1 E106Q or Orai1R91W, but not with a functional Orai1 construct, failed to restore SOCE [7][60][22,60]. Concerning Orai1 splicing variants, an elegant study demonstrated that both variants of Orai1, Orai1α and Orai1β, are equally involved in the generation of ISOC currents in HEK-293 transfected with STIM1, TRPC1 and either Orai1α or Orai1β [43]. This finding suggests that the STIM1-Orai1-TRPC1 complex might include both Orai1α or Orai1β proteins. A model proposed by Cheng and coworkers, in human salivary gland cells, suggests that depletion of intracellular stores promotes Ca2+ influx via Orai1-STIM1 complex, providing a local increase in free Ca2+ concentration that induces the translocation of TRPC1 to the vicinity of the STIM1-Orai1 complex (Figure 2). Beyond the activation of TRPC1 by STIM1, this transition also leads to the association of TRPC1 and Orai1 in the same complex. Interestingly, this model could explain the essential role of Orai1 and the lack of strong evidence supporting the direct association between Orai1 and TRPC1 in the assembly of the STIM1-Orai1-TRPC1 complex [69]. Besides different biophysical properties, the Orai1-STIM1 complex to mediate the ICRAC current and the STIM1-Orai1-TRPC1 ternary complex to mediate the ISOC current also display specific temporal and spatial Ca2+ oscillatory patterns involved in the activation of different physiological functions and in the pathogenesis of a number of diseases (revised in [70]). For instance, Orai1-STIM1-mediated Ca2+ entry promotes the activation and nuclear translocation of the NFAT (nuclear factor of activated T-cells) transcription factor, while a TRPC1-dependent Ca2+ entry is responsible for NF-κB transcription factor activation in human submandibular gland cells [71]. STIM1-Orai1-TRPC1-mediated Ca2+ entry is also required for platelet aggregation [72], insulin release [73], adipocyte differentiation and adiponectin secretion [74], among other functions. Moreover, STIM1-Orai1-TRPC1-dependent Ca2+ currents have been associated to the Ca2+ mobilization responsible for the development of distinct cancer hallmarks in different cancer cell types, including prostate cancer cells [75] and colon cancer cells [76][77][76,77], while STIM1-Orai1-TRPC1-TRPC4-mediated Ca2+ currents are involved in the Ca2+ remodelling involved in hypertrophic cardiomyopathy in rat ventricular myocytes [78]. A more recent study has reported that in anterior pituitary (AP) cells from Orai1-lacking mice TG-induced SOCE as well as Ca2+ entry evoked by TRH and LHRH were impaired, by contrast, SOCE was unaffected in AP cells from mice lacking expression of all seven TRPC channels, although spontaneous intracellular Ca2+-oscillations associated to electrical activity as well as Ca2+ responses to TRH and GHRH were significantly reduced in the absence of TRPC channels, thus suggesting that SOCE might function independently of TRPC channels and that Orai1 and TRPC channels, such as TRPC1, might play different functional roles [79].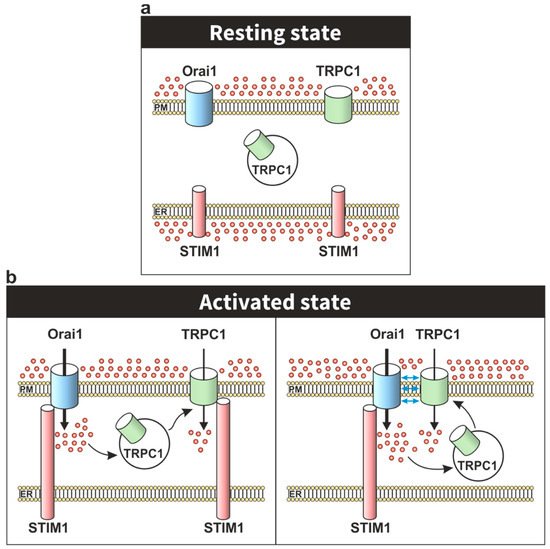
3. Modulation of Orai1 Function by TRPC Channels
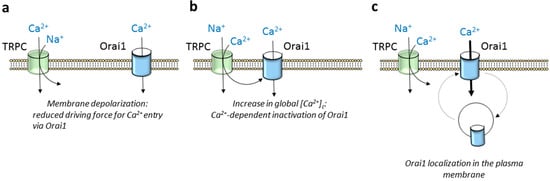
As mentioned previously, TRPC channels, especially TRPC1 [7][58][70][77][22,58,70,77] but also other members of the TRPC subfamily, such as TRPC4 [84][85][84,85] and TRPC6 [86][87][88][89][86,87,88,89], have been reported to conduct Ca2+ entry upon Ca2+ store depletion. However, there is a growing body of evidence indicating that TRPC channels play a more complex role shaping Ca2+ signals through Orai1 channels. TRPC5 and TRPC6 show the greatest selectivity for Ca2+ relative to Na+ of the TRPC subfamily with Ca2+/Na+ permeability ratios around 9 and 5, respectively, while TRPC4 and TRPC1 are approximately equally permeable to Ca2+ and Na+ [90]. The latter means that TRPC channel gating leads to Ca2+ and Na+ influx in favor of an electrochemical gradient, which, in turn, might attenuate the inward flux of Ca2+ through Orai1 channels in two different manners: (1) inducing Ca2+-dependent inactivation of the Orai1 channels and (2) attenuating the driving force for Ca2+ entry as a result of membrane depolarization (Figure 3a,b). Concerning the first issue, fast Ca2+-dependent Orai1 inactivation has been suggested to be evoked by the interaction of Ca2+ entering through the channel itself to cytosolic inactivating sites in close proximity to the channel pore [91][92][91,92]; however, slow inactivation of Orai1 channels is associated to global increases in cytosolic Ca2+ concentration [93] that might be influenced by opening of TRPC channels in the vicinity of Orai1. In tumor cells with a gain of function of TRPC channels, in addition to Ca2+ entry, Na+ influx has been associated to Ca2+ efflux from the mitochondria due to exchange for Na+, thus resulting in further Ca2+-dependent inactivation of Orai1 channels (revised in [94]). Furthermore, the opening of TRPC channels might increase the amount of Ca2+ available to SERCA (sarco/endoplasmic reticulum Ca2+-ATPase) pumps and, therefore, store refilling, thus accelerating the deactivation of Orai1 channels. On the other hand, it has long been reported that TRP channel opening results in membrane depolarization. A well-known depolarizing TRP channel is TRPM4, which has been found to depolarize T lymphocytes [95]. Membrane depolarization induced by TRPC channel gating has been associated to a functional activation of voltage-dependent Ca2+ channels in electrically excitable cells [96][97][96,97]. In addition, depolarization evoked by Ca2+ and Na+ influx through TRPC channels leads to subsequent attenuation of the driving force for Ca2+ entry via Orai1 channels.