Scanning electrochemical microscopy (SECM) is a powerful scanning probe technique for measuring the in situ electrochemical reactions occurring at various sample interfaces, such as the liquid-liquid, solid-liquid, and liquid-gas. The tip/probe of SECM is usually an ultramicroelectrode (UME) or a nanoelectrode that can move towards or over the sample of interest controlled by a precise motor positioning system. Remarkably, electrocatalysts play a crucial role in addressing the surge in global energy consumption by providing sustainable alternative energy sources. Therefore, the precise measurement of catalytic reactions offers profound insights for designing novel catalysts as well as for enhancing their performance. SECM proves to be an excellent tool for characterization and screening catalysts as the probe can rapidly scan along one direction over the sample array containing a large number of different compositions. These features make SECM more appealing than other conventional methodologies for assessing bulk solutions. SECM can be employed for investigating numerous catalytic reactions including the oxygen reduction reaction (ORR), oxygen evolution reaction (OER), hydrogen evolution reaction (HER), water oxidation, glucose oxidation reaction (GOR), and CO2 reduction reaction (CO2RR) with high spatial resolution. Moreover, for improving the catalyst design, several SECM modes can be applied based on the catalytic reactions under evaluation.
- scanning electrochemical microscopy
- ultramicroelectrode
- catalyst
- electrocatalysis
1. Introduction
Advances in electrochemical technologies have played a crucial role in gaining deeper insights into the electrochemical reactions occurring at solid-liquid, liquid-liquid as well as liquid-gas interfaces [1][2][3]. Electrochemical techniques have gained worldwide interest over the recent years as they offer an efficient platform to study the renewable and sustainable energy generation arising from various electrocatalytic chemical reactions, such as photoelectrochemical (PEC) splitting of water, the electrochemical production of H2O2, the electroreduction of CO2, glucose-based biofuel cells (GFCs) for assessing electrocatalytic glucose oxidation, and water splitting reactions [4][5][6][7][8][9][10].
Scanning probe microscopy (SPM) techniques, especially scanning electrochemical microscopy (SECM), can be utilized for the screening of a large number of electrocatalysts and instantaneous in-situ product analysis [11][12]. SECM comprises of an ultramicroelectrode (UME) or a nanoelectrode (NE) acting as the probe (depending on the sample sizes) that enables the efficient mapping of localized electrochemical activity over various homogeneous and heterogeneous surfaces [13][14]. The catalytic reactions may occur heterogeneously on the sample surfaces, and thus SECM can be used to understand the reaction sites, nature of spatial variations, and reaction dynamics of the catalytic systems. Table 1 summarizes the recent advances of SECM for evaluating catalysts. For instance, SECM can measure a variety of localized catalytic redox reactions, including oxygen reduction reaction (ORR) [15][16][17], oxygen evolution reaction (OER) [18][19], hydrogen evolution reaction (HER) [15][20][21][22], water oxidation [23], glucose oxidation reaction (GOR) [24][25], and CO2 reduction reaction (CO2RR) [26][27]. Furthermore, SECM can be employed for the determination of short-lived intermediates (such as CO2−) [28].
Table 1.Recent advances in the applications of SECM for assessing catalysts.
| Instrumentation | Material of the Probe | Mode | Catalysts | Catalytic Reaction | References |
|---|---|---|---|---|---|
| SECM | Pt | RC | PdW nanoparticles supported on nitrogen and sulfur co-doped graphene (NSG) | ORR | [29] |
| SECM | Au | SG/TC | Nanowires of silver chloride and bromide (AgClNWs and AgBrNWs) | ORR | [30] |
| SECM | Pt | RC | Octahedral cobalt sulfide (CoS2) | ORR | [31] |
| SECM | Pt | RC | Manganese tungstate (MnWO4) | ORR | [32] |
| AFM-SECM | Pt | SG/TC; Direct mode |
Fe–N doped planar graphite | ORR; H2O2 production | [16] |
| SECM | Pt | SG/TC | nickel and cobalt-based oxides (NiO, Co3O4 and NixCo3-xO4) |
HO2− production in ORR | [9] |
| SECM | Au | FB | Copper nanostructures (CuNSs) |
ORR and CO2 reduction | [33] |
| SECM | Cu and Fe | TG/SC | Copper | ORR; HER; CO2RR | [34] |
| SECM | Pt | SG/TC; RC |
Nitrogen-bearing carbon spheres (NCSs) | ORR; H2O2 production | [35] |
| SECM | Pt | RC | Nanostructured hybrids based on MoSe2 on reduced graphene oxide (rGO) nanosheets |
ORR | [36] |
| SECM | Pt | SG/TC; TG/SC; RC |
Multiwalled Carbon nanotubes (MWCNTs) with cobalt(IX) protoporphyrin (MWCNTs/CoP) |
ORR; H2O2 production | [37] |
| SECM | Pt | RC | ZnCo2O4 on carbon nanotubes (ZnCo2O4/CNTs) and Pt/C | ORR | [38] |
| AFM-SECM | Au-c-Pt tip | SG/TC | Platinum nanoparticles (Pt NPs) |
ORR; H2O2 production | [39] |
| SECM-SICM | Pt coated pyrolytic carbon | SG/TC; RC |
Gold nanoparticles (Au NPs) |
ORR; H2O2 generation | [40] |
| Raman-SECM | Glassy carbon | SG/TC | Lithium intercalated nickel phosphorus trisulfide (NiPS3) | OER | [41] |
| SI-SECM | Au | FB | CoPi nanosheets | Water oxidation, OER | [23] |
| Raman-SECM | Pt | SG/TC | Ni/Fe and Ni thin films | OER | [42] |
| SECM | Pt | SG/TC | reduced graphene oxide supported ZnCo2O4 microsphere (rGO-ZnCo2O4) |
ORR; OER | [43] |
| SECM | Pt | SG/TC | Cobalt-based metalloids (CoxB and CoxP) composites |
ORR, OER; | [44] |
| SECM | Pt | SG/TC | Mesoporous single-atom-doped graphene–carbon nanotube hybrid | ORR; OER | [45] |
| SECM | Pt | SG/TC; FB | MXenes (2D early transition metal carbide) |
HER | [21] |
| SECM | Pt | SG/TC | Iron sulfide (FeS2) nanostructures (1D, wires and 2D, discs) | HER | [46] |
| SECM | Pt | SG/TC | Metal-organic frameworks (CoSx MOFs) |
HER | [15] |
| SECM | Pt | SG/TC | Sulfur vacancies on Molybdenum disulfide (SV-MoS2) | HER | [47] |
| SECM | Pt | FB; SG/TC |
Mixed-phase MoS2 nanosheets | HER | [48] |
| SECM | Pt | SG/TC | Ni and Ni/α-Ni(OH)2 heterostructures | HER, OER | [49] |
| SECM | Pt | SG/TC | Decamethylruthenocene (DMRc) in 1,2-dichloroethane/water (DCE|W) biphasic system | HER | [50] |
| SECM | Carbon; Pt | FB; SG/TC |
Silver nanoparticles (Ag NPs) and silver nanoclusters (Ag NCs) on multi-wall carbon nanotubes (MWCNT) | Bicarbonate reduction | [51] |
| SECM | Hg/Pt | TG/SC | Au metal surface | Intermediate of CO2 reduction | [52] |
| Instrumentation | Material of the Probe | Mode | Catalysts | Catalytic Reaction | References |
| SECM | Pt | RC | PdW nanoparticles supported on nitrogen and sulfur co-doped graphene (NSG) | ORR | [29] |
| SECM | Au | SG/TC | Nanowires of silver chloride and bromide (AgClNWs and AgBrNWs) | ORR | [30] |
| SECM | Pt | RC | Octahedral cobalt sulfide (CoS2) | ORR | [31] |
| SECM | Pt | RC | Manganese tungstate (MnWO4) | ORR | [32] |
| AFM-SECM | Pt | SG/TC; Direct mode |
Fe–N doped planar graphite | ORR; H2O2 production | [16] |
| SECM | Pt | SG/TC | nickel and cobalt-based oxides (NiO, Co3O4 and NixCo3-xO4) |
HO2− production in ORR | [9] |
| SECM | Au | FB | Copper nanostructures (CuNSs) |
ORR and CO2 reduction | [33] |
| SECM | Cu and Fe | TG/SC | Copper | ORR; HER; CO2RR | [34] |
| SECM | Pt | SG/TC; RC |
Nitrogen-bearing carbon spheres (NCSs) | ORR; H2O2 production | [35] |
| SECM | Pt | RC | Nanostructured hybrids based on MoSe2 on reduced graphene oxide (rGO) nanosheets |
ORR | [36] |
| SECM | Pt | SG/TC; TG/SC; RC |
Multiwalled Carbon nanotubes (MWCNTs) with cobalt(IX) protoporphyrin (MWCNTs/CoP) |
ORR; H2O2 production | [37] |
| SECM | Pt | RC | ZnCo2O4 on carbon nanotubes (ZnCo2O4/CNTs) and Pt/C | ORR | [38] |
| AFM-SECM | Au-c-Pt tip | SG/TC | Platinum nanoparticles (Pt NPs) |
ORR; H2O2 production | [39] |
| SECM-SICM | Pt coated pyrolytic carbon | SG/TC; RC |
Gold nanoparticles (Au NPs) |
ORR; H2O2 generation | [40] |
| Raman-SECM | Glassy carbon | SG/TC | Lithium intercalated nickel phosphorus trisulfide (NiPS3) | OER | [41] |
| SI-SECM | Au | FB | CoPi nanosheets | Water oxidation, OER | [23] |
| Raman-SECM | Pt | SG/TC | Ni/Fe and Ni thin films | OER | [42] |
| SECM | Pt | SG/TC | reduced graphene oxide supported ZnCo2O4 microsphere (rGO-ZnCo2O4) |
ORR; OER | [43] |
| SECM | Pt | SG/TC | Cobalt-based metalloids (CoxB and CoxP) composites |
ORR, OER; | [44] |
| SECM | Pt | SG/TC | Mesoporous single-atom-doped graphene–carbon nanotube hybrid | ORR; OER | [45] |
| SECM | Pt | SG/TC; FB | MXenes (2D early transition metal carbide) |
HER | [21] |
| SECM | Pt | SG/TC | Iron sulfide (FeS2) nanostructures (1D, wires and 2D, discs) | HER | [46] |
| SECM | Pt | SG/TC | Metal-organic frameworks (CoSx MOFs) |
HER | [15] |
| SECM | Pt | SG/TC | Sulfur vacancies on Molybdenum disulfide (SV-MoS2) | HER | [47] |
| SECM | Pt | FB; SG/TC |
Mixed-phase MoS2 nanosheets | HER | [48] |
| SECM | Pt | SG/TC | Ni and Ni/α-Ni(OH)2 heterostructures | HER, OER | [49] |
| SECM | Pt | SG/TC | Decamethylruthenocene (DMRc) in 1,2-dichloroethane/water (DCE|W) biphasic system | HER | [50] |
| SECM | Carbon; Pt | FB; SG/TC |
Silver nanoparticles (Ag NPs) and silver nanoclusters (Ag NCs) on multi-wall carbon nanotubes (MWCNT) | Bicarbonate reduction | [51] |
| SECM | Hg/Pt | TG/SC | Au metal surface | Intermediate of CO2 reduction | [52] |
Depending on the type of electrocatalysis and catalyst used, SECM can operate via various working modes, such as substrate-generation/tip-collection (SG/TC) mode, tip-generation/sample-collection (TG/SC) mode, feedback (FB) mode, redox competition (RC) mode, and direct mode. SECM acts as an ideal tool for assessing the catalytic/electrocatalytic reactions as well as for determining the catalytic efficiency of electrocatalysts based on numerous features, including the electron transfer mechanism, oxidation potential, current density, surface morphology, electronic band structures, porosity, tunability, and availability of active edge sites [11]. High-precision and real-time monitoring of localized catalytic electrochemical reactions can provide a remedy to numerous biotechnological problems such as corrosion of polymers and carbon-based materials, the decay of biofuel cells, etc. [11][12]. The understanding of these chemical reactions may accelerate the development of new technologies and guide the scientists to conduct more precise research [53][54].
2. Applications of SECM
2.1. The Study of Oxygen Reduction Reaction (ORR) by SECM
In order to gain insights into the reactions occurring at the surface of these nanomaterials, the scanning electrochemical microscopy-atomic force microscopy (SECM-AFM) was used to map the catalytic currents with ultra-high resolution (around 50 nm). FigureFigure 3 1 demonstrates that the combination of SECM and AFM can reveal the Fe-coordinated nitrogen sites formed both in the edge as well as basal planes of highly ordered pyrolytic graphite (HOPG) and meanwhile, provides the topographical information on a nanometric scale. The Kolagatla group compared the HOPG surface with ammonia plasma-treated HOPG (N-HOPG), and iron and ammonia plasma-treated HOPG (Fe-N-HOPG) surfaces. They demonstrated that the edge planes of Fe-N-HOPG have enhanced catalytically active sites as compared to other tested surfaces, as the catalytic activity of the ORR in acidic solution arises mainly through the Fe coordinated ‘N’ sites, associated with structural defects in the HOPG surface [16]. This system enables a deeper understanding of the catalytic sites on different composites and thus, paves a way for a better design of the catalysts.
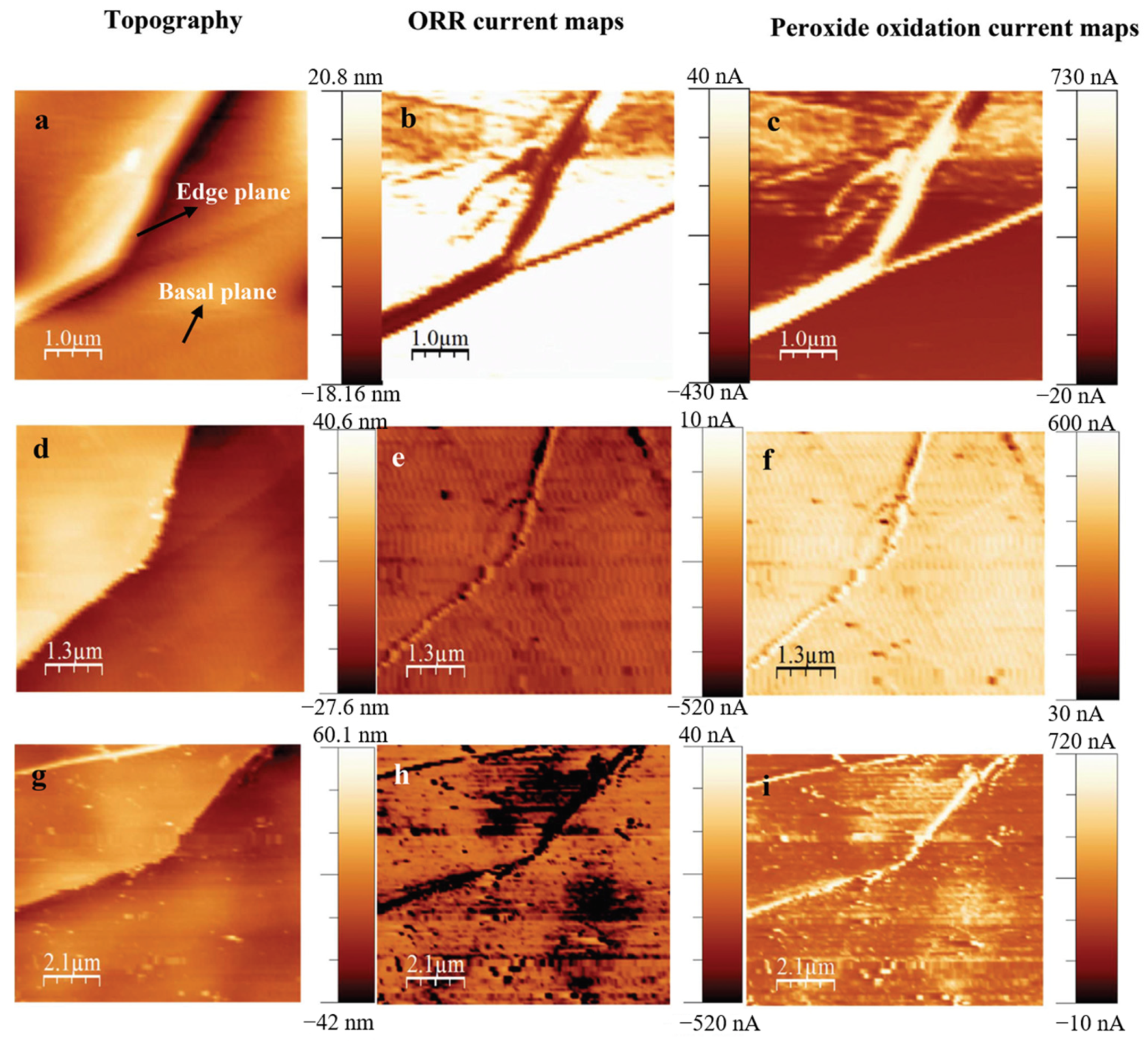
Figure 13. The AFM topography scan, oxygen reduction and peroxide oxidation current mapping images (in this order) of HOPG (a–c), N-HOPG (d–f), and Fe-N-HOPG (g–i), respectively. The applied substrate potentials for the oxygen reduction current mapping are 0.68 V (HOPG and N-HOPG) and 0.7 V (Fe-N-HOPG). The applied tip potential for the hydrogen peroxide oxidation current mapping is 1.2 V. The SECM-AFM tip scan rate used for the current-mapping experiments is 15 ms per point; (1 ms per point = 4 nm ms−1) [16].
2.2. The Study of Oxygen Evolution Reaction (OER) by SECM
The oxygen evolution reaction (OER) is the process of generating oxygen molecules through a chemical reaction. Oxygen molecules can be generated from the electrolysis of water, oxidation of water during oxygenic photosynthesis, or electrocatalytic oxygen evolution reactions [12][19][41][42][43][45][49][55]. In the fields related to metal-air batteries and solar fuel production or other renewable energy technologies, the catalysts for the OER reaction are usually composed of manganese oxides (MnO), ruthenium, iridium oxides, and other first-row metals; however, they are costly and rare [55].
SECM can reveal the OER status in real-time and provide useful information on the reaction rate. In a recent study of OER, Bard’s group used surface interrogation scanning electrochemical microscopy (SI-SECM) to investigate manganese oxidation state on the two electrodeposited manganese-based electrocatalysts, amorphous MnOx, and perovskite CaMnO3. The workflow of the detection of the different manganese oxidation states on the two catalysts is presented in Figure 2Figure 5. The intermediates of the OER process were generated by giving a voltage pulse on the substrate from the open circuit potential (OCP) to a certain potential for several seconds [19]. Consequently, the SECM probe enhanced the production of redox species that titrates adsorbed molecules on the substrate. During the OER process, MnV species were identified in both electrocatalysts by analyzing the feedback currents and finally, the reaction rates calculated. Besides, the Bron group coupled Raman spectroscopy with SECM and demonstrated that in situ Raman-SECM can be utilized for mapping the OER activity of electrochemically deposited Ni and Ni/Fe thin-film electrodes in an alkaline media. This in situ spectroelectrochemical methodology can provide clearer insights into the structure, structural changes, and resulting catalytic activity of NiFe oxides/hydroxides using Raman spectroscopy as well as simultaneously assess the onset of OER using SECM in SG/TC mode at the same location [42].
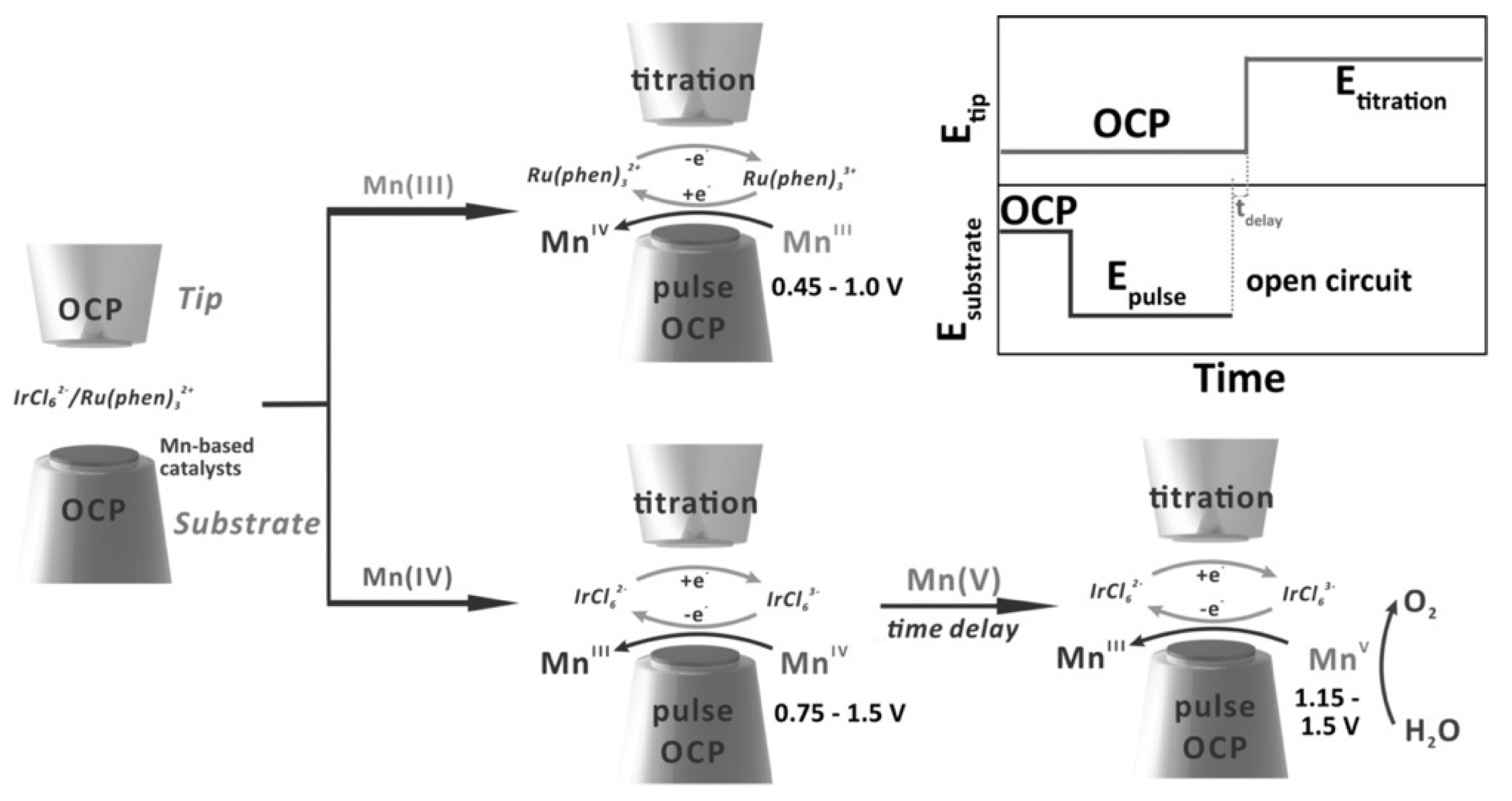
Figure 25. A systematic methodology for SI-SECM. The oxidation and reduction titrations were performed in two separate ways: one for Mn(III) using the redox titrant Ru(phen)32+/3+, and another for Mn(IV) as well as Mn(V) using IrCl62−/3−. Reprinted from ref. [19]. Copyright 2020, John Wiley and Sons [19].
2.3. The Study of Hydrogen Evolution Reaction (HER) by SECM
Hydrogen is a promising alternative energy source that is more environmentally friendly and renewable. Therefore, the development of electrocatalysts for the hydrogen evolution reaction (HER) is an important concern. Typically, catalysts for HER reaction are composed of noble metals. In recent years, SECM has been used for screening earth-abundant electrocatalysts, so the design of active non-precious metal-based catalysts can be improved.
Recently, single-atom catalysts (SACs) with the atomic distribution of metal active sites attracted great attention because of their extraordinary features of high reactivity. The characterization of SACs and the unveiling of catalytic mechanisms on individual sites can be done by the SECM [56]. However, the scanning of a single molecule or single nanoparticle catalysts is relatively feasible. Figure 3Figure 6 presents that Mirkin’s group revealed the HER reaction occurs on a single Au nanoparticle on a polyphenylene film. The current distribution in the SECM image under SG/TC mode reflects the catalytic site of the nanoparticle (Figure 4Figure 7).
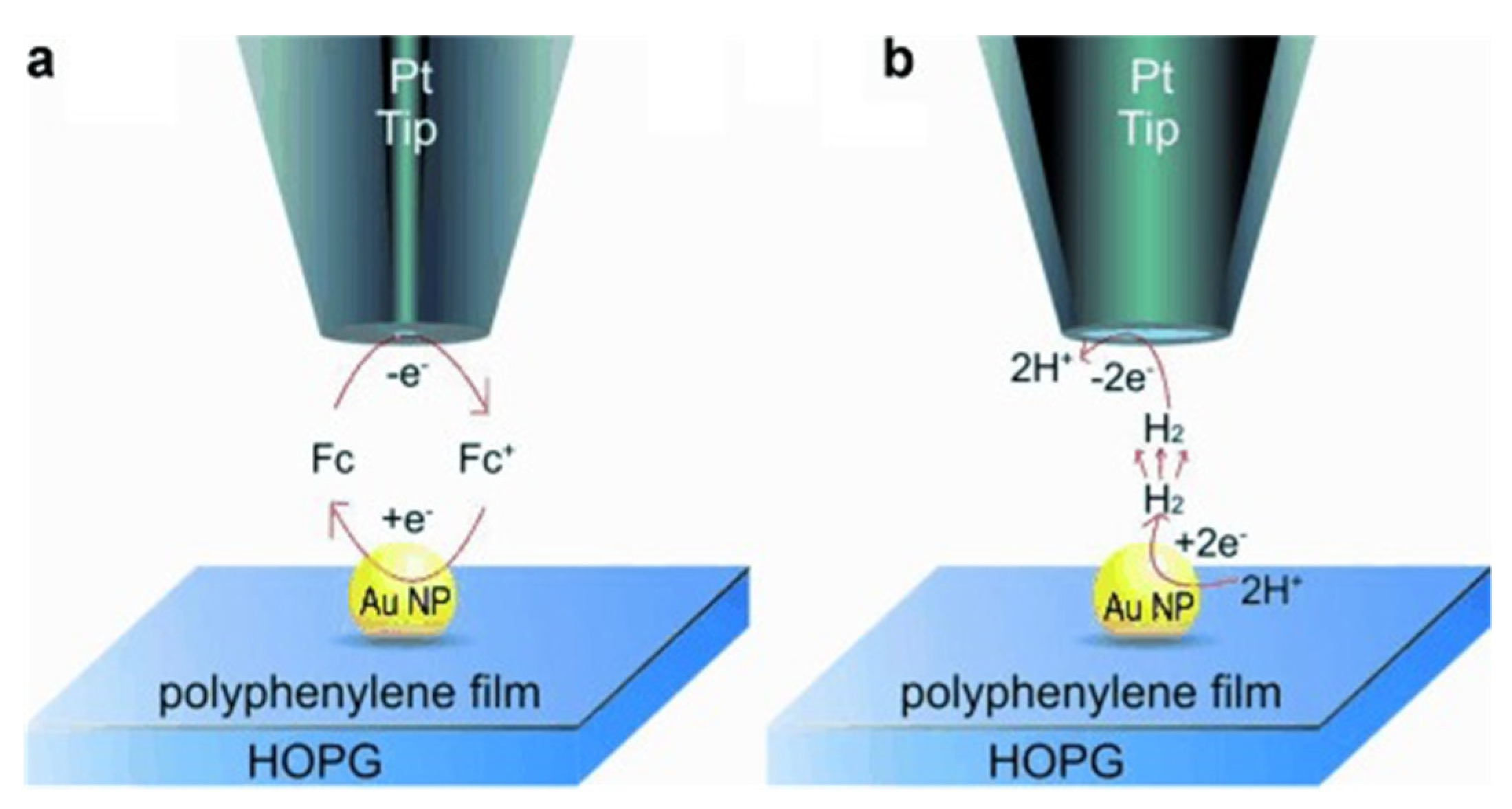
Figure 36. Illustration for SECM being operated on individual Au NPs via: (a) feedback (FB) mode, and (b) SG/TC mode. Reprinted from ref. [57]. Copyright 2014, John Wiley and Sons [58].
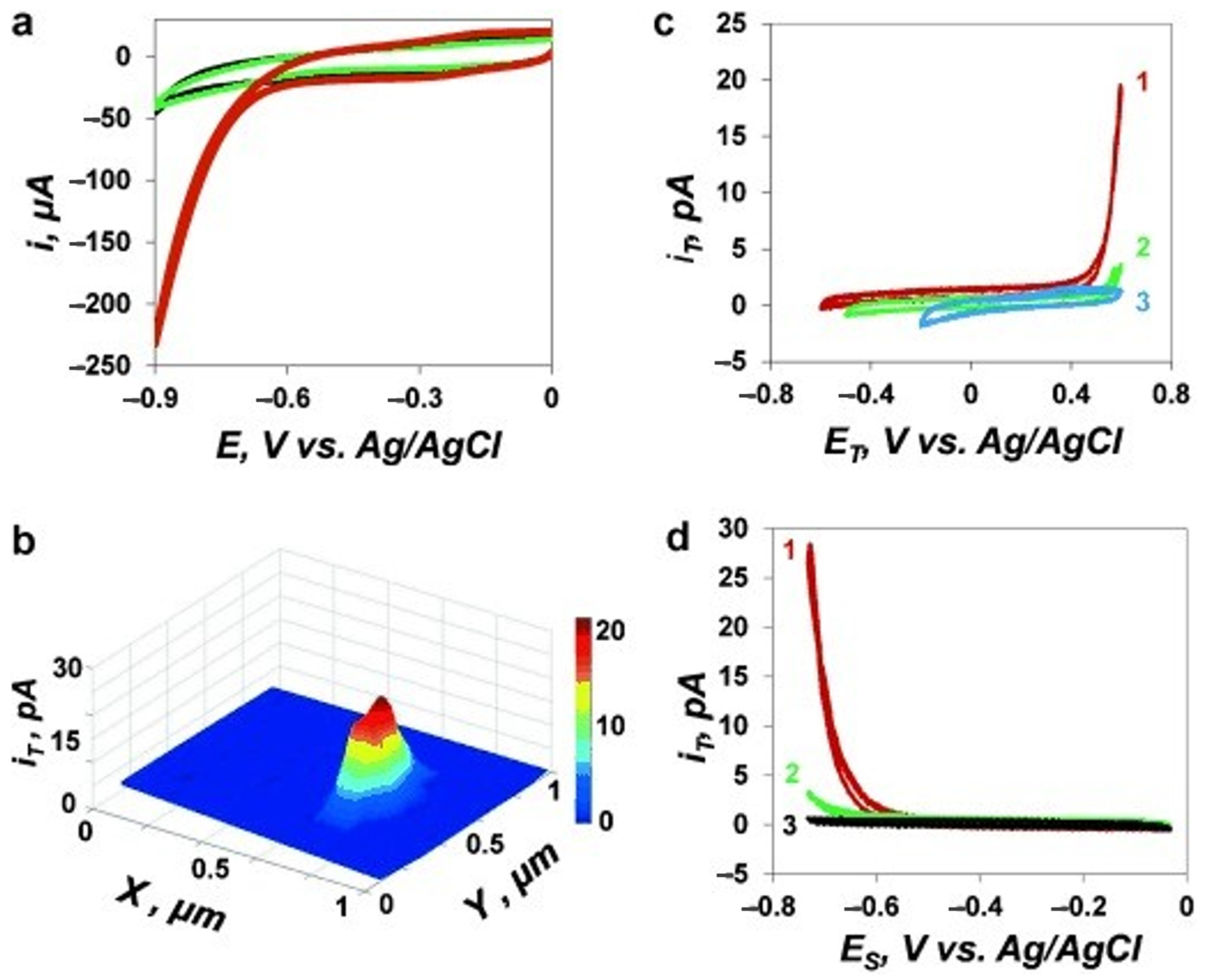
Figure 47. Voltammetric and SECM analysis for assessing the HER activity at gold nanoparticles (AuNPs). (a) Voltammograms corresponding to the reduction of protons at uncoated-HOPG (black), polyphenylene-coated HOPG (green), and 20-nm-gold nanoparticles (AuNPs) immobilized onto the modified HOPG (red). (b) SG/TC map corresponding to the HER activity at an individual 20-nm-gold nanoparticles acquired using a 15 nm-radius Pt tip. ET = 500 mV, ES = −750 mV vs. Ag/AgCl. (c) Voltammograms obtained at a 60 nm-radius Pt tip kept above the AuNP, 80 nm apart from the modified HOPG surface. ET was scanned, and ES (in mV) was −600 (1), −500 (2), and −100 (3). (d) Tip/substrate voltammograms were acquired at the same position as in (c). ES was scanned, and ET (in mV) was 500 (1), 400 (2), and 100 (3). The electrolyte solution comprised 10 mM HClO4 and 0.1 M NaClO4. The potential sweep rate was 100 mV s−1. Reprinted from ref. [58]. Copyright 2014, John Wiley and Sons.
Moreover, novel catalysts such as metal-organic frameworks (MOF) were explored, and the HER mechanism was also interpreted by SECM. The Hod group developed the electrochemically converted-MOFs (termed “EC-MOFs”) into highly active electrocatalysts and optimized their conversion parameters (scan rate, potential range, and number of potential scans) for enhancing their electrochemical performance. They employed SECM in SG-TC mode to map in situ HER activity of the EC-MOF of ZIF-67 into patterned CoSx, an HER electrocatalyst. Furthermore, they also performed in situ OER analysis of a bi-metallic (Fe,Ni)-MIL-53 MOF into patterned FeNiSx, revealing that SECM can serve as a useful technique for optimization of localized catalyst fabrication as well as for designing MOF-based patterned arrays for electrochemical studies [15].
2.4. The Emerging Applications of SECM
In addition to the reactions mentioned above, other catalytic reactions including CO2 reduction, hydrogen peroxide production, and water oxidation, have been investigated by SECM. For instance, various methods for the elimination of greenhouse gases include energy-driven CO2 reduction, photothermal, thermal-catalytic, and artificial photosynthesis. Catalysts used for CO2 reduction reactions determine the efficiency of the conversion of CO2 toward high-value hydrocarbons [23][26][27][59].
References
- Daviddi, E.; Shkirskiy, V.; Kirkman, P.M.; Robin, M.P.; Bentley, C.L.; Unwin, P.R. Nanoscale electrochemistry in a copper/aqueous/oil three-phase system: Surface structure–activity-corrosion potential relationships. Chem. Sci. 2021, 12, 3055–3069.
- Wang, S.; Yang, X.; Wu, F.; Min, L.; Chen, X.; Hou, X. Inner Surface Design of Functional Microchannels for Microscale Flow Control. Small 2019, 16, e1905318.
- Su, H.; Zhou, W.; Zhang, H.; Zhou, W.; Zhao, X.; Li, Y.; Liu, M.; Cheng, W.; Liu, Q. Dynamic Evolution of Solid–Liquid Electrochemical Interfaces over Single-Atom Active Sites. J. Am. Chem. Soc. 2020, 142, 12306–12313.
- Feng, J.; Huang, H.; Yan, S.; Luo, W.; Yu, T.; Li, Z.; Zou, Z. Non-oxide semiconductors for artificial photosynthesis: Progress on photoelectrochemical water splitting and carbon dioxide reduction. Nano Today 2020, 30, 100830.
- Suk, M.; Chung, M.W.; Han, M.H.; Oh, H.-S.; Choi, C.H. Selective H2O2 production on surface-oxidized metal-nitrogen-carbon electrocatalysts. Catal. Today 2021, 359, 99–105.
- Von Kurnatowski, M.; Bortz, M. Modeling and Multi-Criteria Optimization of a Process for H2O2 Electrosynthesis. Processes 2021, 9, 399.
- Ye, W.; Guo, X.; Ma, T. A review on electrochemical synthesized copper-based catalysts for electrochemical reduction of CO2 to C2+ products. Chem. Eng. J. 2021, 414, 128825.
- Boutin, E.; Robert, M. Molecular Electrochemical Reduction of CO2 beyond Two Electrons. Trends Chem. 2021, 3, 359–372.
- Sidhureddy, B.; Prins, S.; Wen, J.; Thiruppathi, A.R.; Govindhan, M.; Chen, A. Synthesis and Electrochemical Study of Mesoporous Nickel-Cobalt Oxides for Efficient Oxygen Reduction. ACS Appl. Mater. Interfaces 2019, 11, 18295–18304.
- Ramanavicius, S.; Ramanavicius, A. Charge Transfer and Biocompatibility Aspects in Conducting Polymer-Based Enzymatic Biosensors and Biofuel Cells. Nanomaterials 2021, 11, 371.
- Limani, N.; Boudet, A.; Blanchard, N.; Jousselme, B.; Cornut, R. Local probe investigation of electrocatalytic activity. Chem. Sci. 2021, 12, 71–98.
- Bertoncello, P. Advances on scanning electrochemical microscopy (SECM) for energy. Energy Environ. Sci. 2010, 3, 1620–1633.
- Kim, J.; Kim, B.-K.; Cho, S.K.; Bard, A.J. Tunneling Ultramicroelectrode: Nanoelectrodes and Nanoparticle Collisions. J. Am. Chem. Soc. 2014, 136, 8173–8176.
- Amatore, C.; Pebay, C.; Thouin, L.; Wang, A.; Warkocz, J.-S. Difference between Ultramicroelectrodes and Microelectrodes: Influence of Natural Convection. Anal. Chem. 2010, 82, 6933–6939.
- Liberman, I.; He, W.; Shimoni, R.; Ifraemov, R.; Hod, I. Spatially confined electrochemical conversion of metal-organic frameworks into metal-sulfides and their in situ electrocatalytic investigation via scanning electrochemical microscopy. Chem. Sci. 2019, 11, 180–185.
- Kolagatla, S.; Subramanian, P.; Schechter, A. Nanoscale mapping of catalytic hotspots on Fe, N-modified HOPG by scanning electrochemical microscopy-atomic force microscopy. Nanoscale 2018, 10, 6962–6970.
- Rastgar, S.; Santos, K.T.; Angelucci, C.A.; Wittstock, G. Catalytic Activity of Alkali Metal Cations for the Chemical Oxygen Reduction Reaction in a Biphasic Liquid System Probed by Scanning Electrochemical Microscopy. Chem. A Eur. J. 2020, 26, 10882–10890.
- Yu, Z. In-Situ and Real-Time Monitoring of Oxygen Evolution during Kolbe Reaction by Scanning Electrochemical Microscopy. Int. J. Electrochem. Sci. 2021, 16, 210240.
- Jin, Z.; Bard, A.J. Surface Interrogation of Electrodeposited MnOx and CaMnO3 Perovskites by Scanning Electrochemical Microscopy: Probing Active Sites and Kinetics for the Oxygen Evolution Reaction. Angew. Chem. Int. Ed. 2021, 60, 794–799.
- Kumar, S.; Sahoo, P.K.; Satpati, A.K. Electrochemical and SECM Investigation of MoS2/GO and MoS2/rGO Nanocomposite Materials for HER Electrocatalysis. ACS Omega 2017, 2, 7532–7545.
- Djire, A.; Wang, X.; Xiao, C.; Nwamba, O.C.; Mirkin, M.V.; Neale, N.R. Basal Plane Hydrogen Evolution Activity from Mixed Metal Nitride MXenes Measured by Scanning Electrochemical Microscopy. Adv. Funct. Mater. 2020, 30, 2001136.
- Iffelsberger, C.; Ng, S.; Pumera, M. Catalyst coating of 3D printed structures via electrochemical deposition: Case of the transition metal chalcogenide MoSx for hydrogen evolution reaction. Appl. Mater. Today 2020, 20, 100654.
- Ahn, H.S.; Bard, A.J. Surface Interrogation of CoPi Water Oxidation Catalyst by Scanning Electrochemical Microscopy. J. Am. Chem. Soc. 2015, 137, 612–615.
- Soldà, A.; Valenti, G.; Marcaccio, M.; Giorgio, M.; Pelicci, P.G.; Paolucci, F.; Rapino, S. Glucose and Lactate Miniaturized Biosensors for SECM-Based High-Spatial Resolution Analysis: A Comparative Study. ACS Sensors 2017, 2, 1310–1318.
- Jayathilake, N.M.; Koley, D. Glucose Microsensor with Covalently Immobilized Glucose Oxidase for Probing Bacterial Glucose Uptake by Scanning Electrochemical Microscopy. Anal. Chem. 2020, 92, 3589–3597.
- Mayer, F.D.; Hosseini-Benhangi, P.; Sánchez-Sánchez, C.M.; Asselin, E.; Gyenge, E.L. Scanning electrochemical microscopy screening of CO2 electroreduction activities and product selectivities of catalyst arrays. Commun. Chem. 2020, 3, 1–9.
- Strange, L.E.; Yadav, J.; Li, X.; Pan, S. Editors’ Choice—Review—Creating Electrocatalytic Heterojunctions for Efficient Photoelectrochemical CO2 Reduction to Chemical Fuels. J. Electrochem. Soc. 2020, 167, 146518.
- Wittstock, G.; Burchardt, M.; Pust, S.E.; Shen, Y.; Zhao, C. Scanning Electrochemical Microscopy for Direct Imaging of Reaction Rates. Angew. Chem. Int. Ed. 2007, 46, 1584–1617.
- Sun, X.; Li, W.; Mi, H.; Li, Y.; Zhang, P.; Ren, X. Nitrogen and sulfur co-doped graphene supported PdW alloys as highly active electrocatalysts for oxygen reduction reaction. Int. J. Hydrogen Energy 2018, 43, 5530–5540.
- Kim, S.-J.; Lee, S.-C.; Lee, C.; Kim, M.H.; Lee, Y. Evolution of silver to a better electrocatalyst: Water-assisted oxygen reduction reaction at silver chloride nanowires in alkaline solution. Nano Energy 2018, 48, 134–143.
- Singh, V.; Tiwari, A.; Nagaiah, T.C. Facet-controlled morphology of cobalt disulfide towards enhanced oxygen reduction reaction. J. Mater. Chem. A 2018, 6, 22545–22554.
- Tiwari, A.; Singh, V.; Nagaiah, T.C. Tuning the MnWO4 morphology and its electrocatalytic activity towards oxygen reduction reaction. J. Mater. Chem. A 2018, 6, 2681–2692.
- Michalak, M.; Roguska, A.; Nogala, W.; Opallo, M. Patterning Cu nanostructures tailored for CO2 reduction to electrooxidizable fuels and oxygen reduction in alkaline media. Nanoscale Adv. 2019, 1, 2645–2653.
- Zhang, Q.; Liu, P.; Zhu, Z.; Zhang, J.; Cao, F. The study of the H2O2 during oxygen reduction process on typically corroding metal surface using tip generation-substrate collection mode of SECM. Corros. Sci. 2020, 164, 108312.
- Tiwari, A.; Singh, V.; Mandal, D.; Nagaiah, T.C. Nitrogen containing carbon spheres as an efficient electrocatalyst for oxygen reduction: Microelectrochemical investigation and visualization. J. Mater. Chem. A 2017, 5, 20014–20023.
- Xin, S.; Liu, Z.; Ma, L.; Sun, Y.; Xiao, C.; Li, F.; Du, Y. Visualization of the electrocatalytic activity of three-dimensional graphene oxide hybrid nanostructures for oxygen reduction reaction. Nano Res. 2016, 9, 3795–3811.
- Dobrzeniecka, A.; Zeradjanin, A.R.; Masa, J.; Blicharska, M.; Wintrich, D.; Kulesza, P.J.; Schuhmann, W. Evaluation of kinetic constants on porous, non-noble catalyst layers for oxygen reduction—A comparative study between SECM and hydrodynamic methods. Catal. Today 2016, 262, 74–81.
- Ma, L.; Zhou, H.; Xin, S.; Xiao, C.; Li, F.; Ding, S. Characterization of local electrocatalytical activity of nanosheet-structured ZnCo2O4/carbon nanotubes composite for oxygen reduction reaction with scanning electrochemical microscopy. Electrochim. Acta 2015, 178, 767–777.
- Kolagatla, S.; Subramanian, P.; Schechter, A. Simultaneous Mapping of Oxygen Reduction Activity and Hydrogen Peroxide Generation on Electrocatalytic Surfaces. ChemSusChem 2019, 12, 2708–2714.
- O’Connell, M.A.; Lewis, J.R.; Wain, A.J. Electrochemical imaging of hydrogen peroxide generation at individual gold nanoparticles. Chem. Commun. 2015, 51, 10314–10317.
- Konkena, B.; Masa, J.; Botz, A.J.R.; Sinev, I.; Xia, W.; Koßmann, J.; Drautz, R.; Muhler, M.; Schuhmann, W. Metallic Core–Shell Heterostructures as Highly Efficient and Stable Electrocatalyst for the Oxygen Evolution Reaction. ACS Catal. 2017, 7, 229–237.
- Steimecke, M.; Seiffarth, G.; Bron, M. In Situ Characterization of Ni and Ni/Fe Thin Film Electrodes for Oxygen Evolution in Alkaline Media by a Raman-Coupled Scanning Electrochemical Microscope Setup. Anal. Chem. 2017, 89, 10679–10686.
- Chakrabarty, S.; Mukherjee, A.; Su, W.-N.; Basu, S. Improved bi-functional ORR and OER catalytic activity of reduced graphene oxide supported ZnCo2O4 microsphere. Int. J. Hydrogen Energy 2019, 44, 1565–1578.
- Barwe, S.; Andronescu, C.; Engels, R.; Conzuelo, F.; Seisel, S.; Wilde, P.; Chen, Y.-T.; Masa, J.; Schuhmann, W. Cobalt metalloid and polybenzoxazine derived composites for bifunctional oxygen electrocatalysis. Electrochim. Acta 2019, 297, 1042–1051.
- Tavakkoli, M.; Flahaut, E.; Peljo, P.; Sainio, J.; Davodi, F.; Lobiak, E.V.; Mustonen, K.; Kauppinen, E.I. Mesoporous Single-Atom-Doped Graphene–Carbon Nanotube Hybrid: Synthesis and Tunable Electrocatalytic Activity for Oxygen Evolution and Reduction Reactions. ACS Catal. 2020, 10, 4647–4658.
- Jasion, D.; Barforoush, J.M.; Qiao, Q.; Zhu, Y.; Ren, S.; Leonard, K.C. Low-Dimensional Hyperthin FeS2 Nanostructures for Efficient and Stable Hydrogen Evolution Electrocatalysis. ACS Catal. 2015, 5, 6653–6657.
- Xiaolin, Z.; Du, M.; Mleczko, M.J.; Koh, A.L.; Nishi, Y.; Pop, E.; Bard, A.J.; Zheng, X. Kinetic Study of Hydrogen Evolution Reaction over Strained MoS2 with Sulfur Vacancies Using Scanning Electrochemical Microscopy. J. Am. Chem. Soc. 2016, 138, 5123–5129.
- Sun, T.; Zhang, H.; Wang, X.; Liu, J.; Xiao, C.; Nanayakkara, S.U.; Blackburn, J.L.; Mirkin, M.V.; Miller, E.M. Nanoscale mapping of hydrogen evolution on metallic and semiconducting MoS2 nanosheets. Nanoscale Horiz. 2018, 4, 619–624.
- Gao, X.; Chen, Y.; Sun, T.; Huang, J.; Zhang, W.; Wang, Q.; Cao, R. Karst landform-featured monolithic electrode for water electrolysis in neutral media. Energy Environ. Sci. 2020, 13, 174–182.
- Jedraszko, J.; Adamiak, W.; Nogala, W.; Girault, H.H.; Opallo, M. SECM study of hydrogen photogeneration in a 1,2-dichloroethane | water biphasic system with decamethylruthenocene electron donor regeneration. J. Electroanal. Chem. 2018, 819, 101–106.
- Arrocha-Arcos, A.; Cervantes-Alcalá, R.; Huerta-Miranda, G.; Miranda-Hernández, M. Electrochemical reduction of Bicarbonate to Formate with Silver Nanoparticles and Silver Nanoclusters supported on Multiwalled Carbon Nanotubes. Electrochim. Acta 2017, 246, 1082–1087.
- Kai, T.; Zhou, M.; Duan, Z.; Henkelman, G.A.; Bard, A.J. Detection of CO2•– in the Electrochemical Reduction of Carbon Dioxide in N,N-Dimethylformamide by Scanning Electrochemical Microscopy. J. Am. Chem. Soc. 2017, 139, 18552–18557.
- Lin, Y.-T.; Darvishi, S.; Preet, A.; Huang, T.-Y.; Lin, S.-H.; Girault, H.H.; Wang, L.; Lin, T.-E. A Review: Electrochemical Biosensors for Oral Cancer. Chemosensors 2020, 8, 54.
- Lin, T.-E.; Rapino, S.; Girault, H.H.; Lesch, A. Electrochemical imaging of cells and tissues. Chem. Sci. 2018, 9, 4546–4554.
- Bergmann, A.; Zaharieva, I.; Dau, H.; Strasser, P. Electrochemical water splitting by layered and 3D cross-linked manganese oxides: Correlating structural motifs and catalytic activity. Energy Environ. Sci. 2013, 6, 2745–2755.
- Li, P.; Jin, Z.; Qian, Y.; Fang, Z.; Xiao, D.; Yu, G. Supramolecular confinement of single Cu atoms in hydrogel frameworks for oxygen reduction electrocatalysis with high atom utilization. Mater. Today 2020, 35, 78–86.
- Salomo, M.; Pust, S.E.; Wittstock, G.; Oesterschulze, E. Integrated cantilever probes for SECM/AFM characterization of surfaces. Microelectron. Eng. 2010, 87, 1537–1539.
- Sun, T.; Yu, Y.; Zacher, B.J.; Mirkin, M.V. Scanning Electrochemical Microscopy of Individual Catalytic Nanoparticles. Angew. Chem. Int. Ed. 2014, 53, 14120–14123.
- Kromer, M.L.; Simpson, B.H.; Rodríguez-López, J. Evaluating the impact of catalyst selection and semiconductor band edge on the photoelectrochemical production of H2O2 via a real-time in situ probe. J. Electroanal. Chem. 2020, 875, 114677.
