The interaction between neurons and a tumor are bilateral and promote metastasis on one hand, and the formation of new nerve structures (neoneurogenesis) on the other. Substances such as neurotransmitters and neurotrophins being the main actors in such interplay, it seems reasonable to expect that alternative splicing and the different populations of protein isoforms can affect tumor-derived neurogenesis.
- neoneurogenesis
- nerves
- alternative splicing
- cancer growth and development
1. The Structure and Signaling of the Autonomic Nervous System
The Autonomic Nervous System (ANS, also called the “visceral nervous system” or the “involuntary nervous system”) is made of the nerves and the ganglia (i.e., nerve clusters) that control body visceral functions below the level of consciousness (such as heart and respiratory rates, digestion and pupillary dilation) [1]. This is made of three sub-systems (
):
-
The sympathetic nervous system (SNS), or “fight or flight” system, which is generically responsible for quick response processes (e.g., vasoconstriction to divert blood flow, dilation of the respiratory ducts, heart rate increase).
-
The parasympathetic nervous system (PNS), or “rest and digest” system, which governs slower responses (e.g., vasodilation for gastro-intestinal functions, stimulation of saliva secretion); and
-
The enteric nervous system (ENS), or “feed and breed” system, which mainly controls the function of the gastrointestinal system.
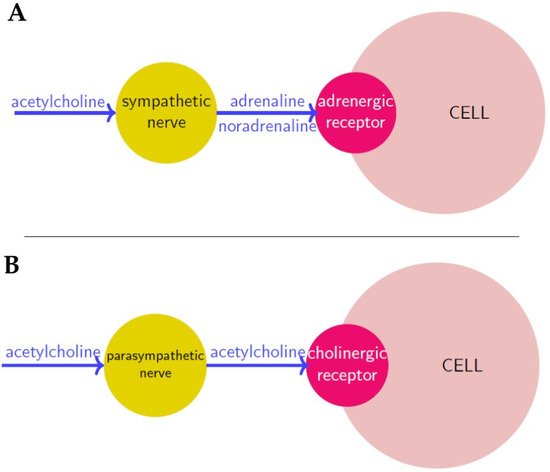
Figure 1.
Neurosignaling in sympathetic (
A
) and parasympathetic (
B
) innervation respectively. Neurotransmitters are in blue, nerves are in yellow and receptors in red.
Nerve cells or neurons propagate information through electro-chemical impulses. These impulses travel along the axons, as such the axons are referred to as
nerve fibres
. Two neurons exchange information across a synapse through chemicals (the so-called
neurotransmitters
), which are a “translation” of the electrical signals travelling inside the neurons. Finally, a neurotransmitter released by a nerve binds to a receptor on another cell, and according to the receptor type, it induces a certain action.
2. Tumor-Nerves Reciprocal Effects
A relationship between tumors and the nervous system has been suspected for a long time (since Galen in the 2nd century AD) [2], because of several observations such as the effects of stress on cancer progression, the high innervation of tumor tissues, or the influence that neurotransmitters have on tumorigenesis [3][4]. New evidence strongly suggests the neuronal system is a key player in cancer initiation, progression and dissemination [4][5]. It is believed that in the same way that the nervous system can influences growth, development, and maintenance in normal tissue [6][7][8], it can contribute to the development and spread of cancer [4][9][10]. As such the formation of new neural tissue has been identified as a hallmark of cancer and can be correlated with cancer severity [11][12]. The connection between the ANS and tumors is bilateral, in the sense that on one hand tumor cells produce factors that induce the formation of a neural networks, a process called neoneurogenesis [13] and on the other hand the newly formed nerves release neurotransmitters that affect tumor growth and migration [14][15]. For example, many cancer patients exhibit signs of stress and depression, that have an effect on the immune system and tumor growth [16]. The (direct) interaction between peripheral nerve cells and tumor cells is usually called the
A relationship between tumors and the nervous system has been suspected for a long time (since Galen in the 2nd century AD) [3], because of several observations such as the effects of stress on cancer progression, the high innervation of tumor tissues, or the influence that neurotransmitters have on tumorigenesis [4,5]. New evidence strongly suggests the neuronal system is a key player in cancer initiation, progression and dissemination [5,6]. It is believed that in the same way that the nervous system can influences growth, development, and maintenance in normal tissue [7,8,9], it can contribute to the development and spread of cancer [5,10,11]. As such the formation of new neural tissue has been identified as a hallmark of cancer and can be correlated with cancer severity [12,13]. The connection between the ANS and tumors is bilateral, in the sense that on one hand tumor cells produce factors that induce the formation of a neural networks, a process called neoneurogenesis [14] and on the other hand the newly formed nerves release neurotransmitters that affect tumor growth and migration [15,16]. For example, many cancer patients exhibit signs of stress and depression, that have an effect on the immune system and tumor growth [17]. The (direct) interaction between peripheral nerve cells and tumor cells is usually called the
neuro-neoplastic synapse
(
A) [18].
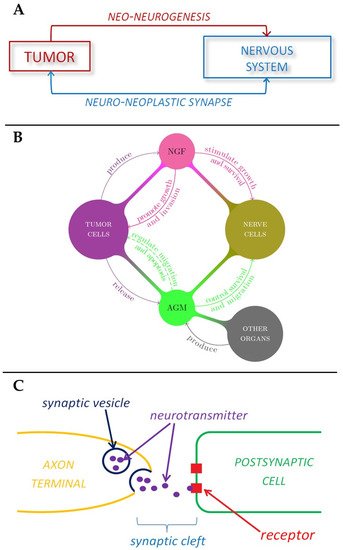
Figure 2.
Schematic representations of the role played by nervous system in tumor development. (
A
) Tumor-nerve bi-directional interaction. (
B
) The role of nerve growth factors and axon guidance molecules in tumor-nerve relationship. (
C
) The mechanism of neurotransmitter signaling in a synapse.
In addition to the formation of nerves supporting the formation of new tissue in the form of tumors, recent evidence has shown that cancer cells are able to actively migrate along nerves, this process is known as
neural tracking and involves the cancer cells migrating along axons [18].
and involves the cancer cells migrating along axons [19].
The invasion of cancer cells along nerves or nerve tissue supporting the growth of cancers around nerves, is known as
perineural invasion (PNI) [18]. This process is most common in cancers affecting organs that are extensively innervated, such as pancreatic cancer, head and neck cancer, prostate cancer and colorectal cancer [19][20]. PNI results in patients experiencing symptoms such as irritated skin, numbness, or paralysis and is an indicator of poor patient survival [21]. Neural bodies that are in close proximity to the tumors (such as post-ganglion neural series) can rapidly be influenced by, responding to, or influencing the tumor microenvironment. The tumor micro-environment is also influenced by the sensory nerves which normally provide information to the central nervous system (CNS) [22]. Therefore, nerve tissue supports the growth and progression of cancer as well as providing a means for cancer to metastasize [4][14][23][24]. However, it is not yet known if the progression of cancer is related to the presence and action of existing neurons located in the region of the tumor or if the progression of metastasis requires the development of new neurons [12].
(PNI) [19]. This process is most common in cancers affecting organs that are extensively innervated, such as pancreatic cancer, head and neck cancer, prostate cancer and colorectal cancer [25,26]. PNI results in patients experiencing symptoms such as irritated skin, numbness, or paralysis and is an indicator of poor patient survival [27]. Neural bodies that are in close proximity to the tumors (such as post-ganglion neural series) can rapidly be influenced by, responding to, or influencing the tumor microenvironment. The tumor micro-environment is also influenced by the sensory nerves which normally provide information to the central nervous system (CNS) [2]. Therefore, nerve tissue supports the growth and progression of cancer as well as providing a means for cancer to metastasize [5,15,28,29]. However, it is not yet known if the progression of cancer is related to the presence and action of existing neurons located in the region of the tumor or if the progression of metastasis requires the development of new neurons [13].
3. The Role of Nerve Growth Factors and Axon Guidance Molecules in Tumor-Neuron Interactions
In general, high levels of innervation in tumors correlate with a poor disease outcome. Indeed, tumor cells have the ability to produce substances that stimulate the growth and survival of nerve cells, such as the Nerve Growth Factor, or NGF, which belongs to a class of growth factors called
neurotrophins [25][26]. These growth factors, together with glial cell-line derived neurotrophic factor family ligands (GFLs) and neuropoietic cytokines, form a family of proteins called
[30,31]. These growth factors, together with glial cell-line derived neurotrophic factor family ligands (GFLs) and neuropoietic cytokines, form a family of proteins called
neurotrophic factors, which control the growth and survival of newly formed neurons and the maintenance of mature ones. These growth factors can promote tumor growth [25] and inhibit aggregation of cancer cells, thus promoting tumor invasion, in a way that is not well understood yet [27]. Interestingly, it has been shown that NGF could also induce the migration of endothelial cells, thus enhancing angiogenesis (
, which control the growth and survival of newly formed neurons and the maintenance of mature ones. These growth factors can promote tumor growth [30] and inhibit aggregation of cancer cells, thus promoting tumor invasion, in a way that is not well understood yet [32]. Interestingly, it has been shown that NGF could also induce the migration of endothelial cells, thus enhancing angiogenesis (
Figure 2B) [28]. In addition to these substances tumors also release axon guidance molecules. The term axon guidance denotes the process by which neurons send out axons along a very precise path in order to reach the correct targets. In fact, the tip of an axon (or
B) [33]. In addition to these substances tumors also release axon guidance molecules. The term axon guidance denotes the process by which neurons send out axons along a very precise path in order to reach the correct targets. In fact, the tip of an axon (or
growth cone
) is equipped with receptors that can sense (gradients of) chemicals, called
guidance cues
, which “tell” them where to expand (
B) [34].
4. ANS Effects on Tumor Cells
Although it has been empirically known for a long time that stress and depression affect the course of cancer, it is only recently that a more solid bio-chemical explanation of this hypothesis was found, after the discovery of the first neurotransmitter in the 20th century by Otto Loevi [30]. Initially, the effect that the nervous system has on cancer development was considered to be only “indirect”, through perineural invasion and modulation of immune function [31]. In fact, neurotransmitters regulate the cytotoxicity of T lymphocytes and natural killer cells [32] and induce leukocyte migration [14][33]. The consequent immunosuppression can favor tumor growth and progression, since it impairs the anti-tumor response [34][35]. Moreover, some neurotransmitters such as histamine, serotonin and angiotensin also induce tumor growth [14]. In summary, the effects that the nervous system has on tumors are either indirect (such as perineural invasion and immunosuppression) or direct (since neurotransmitters induce chemotaxis, migration and growth of cancer cells).
Although it has been empirically known for a long time that stress and depression affect the course of cancer, it is only recently that a more solid bio-chemical explanation of this hypothesis was found, after the discovery of the first neurotransmitter in the 20th century by Otto Loevi [38]. Initially, the effect that the nervous system has on cancer development was considered to be only “indirect”, through perineural invasion and modulation of immune function [39]. In fact, neurotransmitters regulate the cytotoxicity of T lymphocytes and natural killer cells [40] and induce leukocyte migration [15,41]. The consequent immunosuppression can favor tumor growth and progression, since it impairs the anti-tumor response [42,43]. Moreover, some neurotransmitters such as histamine, serotonin and angiotensin also induce tumor growth [15]. In summary, the effects that the nervous system has on tumors are either indirect (such as perineural invasion and immunosuppression) or direct (since neurotransmitters induce chemotaxis, migration and growth of cancer cells).
5. Propinquity with Angiogenesis and Lymphangiogenesis
In summary, angio-, lymphangio- and neoneuro-genesis are alike, in the sense that they have common regulation factors and all together promote metastasis. For instance, tumor cells secrete angiogenic factors (mainly VEGF) which initiate tumor vascularization, but at the same time tumor-innervating nerve cells release neurotransmitters which are proliferative or promigratory signals for the tumor cells. Furthermore, nerve fibers can constitute a kind of “route” for tumor cell to disseminate, perineural invasion, such as blood and lymphatic vessels [14]. Considering all these factors, the hypothesis has been advanced that tumors stimulate their own innervation in a process similar to angiogenesis and lymphangiogenesis. Moreover, neurogenic and angiogenic factors have overlapping functions. For example, in [11] it was reported that NGF has angiogenic effects, and that the axonal attractant netrin-1 is also an angiogenic factor. In turn, VEGF seems to be a chemoattractant for neural progenitors. It is then reasonable to think that more factors are involved in neoneurogenesis, as is the case for angiogenesis.
In summary, angio-, lymphangio- and neoneuro-genesis are alike, in the sense that they have common regulation factors and all together promote metastasis. For instance, tumor cells secrete angiogenic factors (mainly VEGF) which initiate tumor vascularization, but at the same time tumor-innervating nerve cells release neurotransmitters which are proliferative or promigratory signals for the tumor cells. Furthermore, nerve fibers can constitute a kind of “route” for tumor cell to disseminate, perineural invasion, such as blood and lymphatic vessels [15]. Considering all these factors, the hypothesis has been advanced that tumors stimulate their own innervation in a process similar to angiogenesis and lymphangiogenesis. Moreover, neurogenic and angiogenic factors have overlapping functions. For example, in [12] it was reported that NGF has angiogenic effects, and that the axonal attractant netrin-1 is also an angiogenic factor. In turn, VEGF seems to be a chemoattractant for neural progenitors. It is then reasonable to think that more factors are involved in neoneurogenesis, as is the case for angiogenesis.
6. Neurotransmitters and Tumor Cells
It seems that tumors are also able to produce neurotransmitters, maybe (only) after neuroendocrine differentiation. In fact, it has been observed that prostate cancer cells can acquire neuroendocrine characteristics, perhaps as an adaptive reaction against therapeutic agents [36][37][38].
It seems that tumors are also able to produce neurotransmitters, maybe (only) after neuroendocrine differentiation. In fact, it has been observed that prostate cancer cells can acquire neuroendocrine characteristics, perhaps as an adaptive reaction against therapeutic agents [58,59,60].
7. Cancer Development and Stress
The release of neurotransmitters has been found to be altered by stress or depression [39]. This was observed in studies where it was noted that the induction of catecholamine by cellular stress led to increased levels of neurotransmitters such as noradrenaline [40]. This cellular stress stimulus is associated with increased incidence of cancer in a variety of solid tumors, including ovarian, prostate, breast, and pancreatic cancer [41][42][43][44][45].
The release of neurotransmitters has been found to be altered by stress or depression [61]. This was observed in studies where it was noted that the induction of catecholamine by cellular stress led to increased levels of neurotransmitters such as noradrenaline [62]. This cellular stress stimulus is associated with increased incidence of cancer in a variety of solid tumors, including ovarian, prostate, breast, and pancreatic cancer [55,63,64,65,66].
8. Nerve Growth Factor and Alternative Splicing
Nerve Growth Factor (NGF) is a neurotrophic growth factor that binds to two different receptors. One is a high affinity receptor, Tropomyosin-related kinase A (TRKA) while the other is the low affinity receptor p75 neurotrophin receptor (p75NTR). These variants arise from the use of alternate promoters [46]. While both variants promoted neurotrophic responses when both receptors were active, the inhibition of either TrkA of p75NTR demonstrated that these two variants were uniquely related to the activity of the two receptors. ProNGF-A promoted survival of neurons when TrkA was blocked, while ProNGF-B had the effect of causing the cells to differentiate when the p75NTR receptor was blocked. Therefore, the pro or anticancer signaling of these variants may be the result of the ratio of the different receptors on the cell surface, in combination with the different ratio of variants present [47]. Pro-NGF has the added ability to bind to a p75NTR-Sortilin receptor complex, allowing it to initiate pro-apoptotic signaling [48][49].
Nerve Growth Factor (NGF) is a neurotrophic growth factor that binds to two different receptors. One is a high affinity receptor, Tropomyosin-related kinase A (TRKA) while the other is the low affinity receptor p75 neurotrophin receptor (p75NTR). These variants arise from the use of alternate promoters [76]. While both variants promoted neurotrophic responses when both receptors were active, the inhibition of either TrkA of p75NTR demonstrated that these two variants were uniquely related to the activity of the two receptors. ProNGF-A promoted survival of neurons when TrkA was blocked, while ProNGF-B had the effect of causing the cells to differentiate when the p75NTR receptor was blocked. Therefore, the pro or anticancer signaling of these variants may be the result of the ratio of the different receptors on the cell surface, in combination with the different ratio of variants present [77]. Pro-NGF has the added ability to bind to a p75NTR-Sortilin receptor complex, allowing it to initiate pro-apoptotic signaling [78,79].
