L-citrulline (CIT) is an organic compound and a non-essential amino acid [1], and the body can synthesize it endogenously. Diet is a poor source of CIT and endogenous synthesis the its main source in the body. Watermelon is the principal source of that amino acid in the diet; the name citrulline comes from
L-citrulline (CIT) is an organic compound and a non-essential amino acid, and the body can synthesize it endogenously. Diet is a poor source of CIT and endogenous synthesis the its main source in the body. Watermelon is the principal source of that amino acid in the diet; the name citrulline comes from
Citrullus lanatus (Thunb.), the scientific name for watermelon. CIT concentration in watermelon depends on the type of cultivir and usually ranges between 0.7 and 3.6 g/kg of fresh weight [4]. CIT is present in both the flesh and in the ring [5].
(Thunb.), the scientific name for watermelon. CIT concentration in watermelon depends on the type of cultivir and usually ranges between 0.7 and 3.6 g/kg of fresh weight. CIT is present in both the flesh and in the ring.
- functional food
- bio-active compounds
- sport performance
- watermelon
- ergogenic aids
1. Introduction
L-citrulline (CIT) is an organic compound and a non-essential amino acid [1], and the body can synthesize it endogenously. CIT is a non-protein amino acid, which is another main metabolic characteristic; it is not one of the 20 primary amino acids encoded by DNA and, therefore, not involved in protein synthesis [2]. Diet is a poor source of CIT and endogenous synthesis is its main source in the body [3]. In fact, watermelon is the principal source of that amino acid in the diet; the name CIT comes from
Citrullus lanatus
(Thunb.), the scientific name for watermelon. CIT concentration in watermelon depends on the type of cultivar and usually ranges between 0.7 and 3.6 g/kg of fresh weight [4]. CIT is present in both the flesh and in the rind [5]; during watermelon development, there is a progressive accumulation of CIT in those tissues [6], in particular, under stress conditions. In addition, watermelon by-products such as the rind from the fresh-cut watermelon industry can be used for CIT extraction [7], improving the sustainability of the fresh-cut industry, and promoting a circular economy model. World watermelon harvest area and production amounted to 3 million hectares and 100 million tons, respectively, in 2019 [8]. This fruit is mostly appreciated by consumers because of its capability for refreshing them. The United Nations has declared 2021 as the International Year of Fruits and Vegetables. The goal is to raise awareness of the nutritional and health benefits of consuming more fruits and vegetables as part of a diversified, balanced, and healthy diet and lifestyle, as well as to direct policy attention to reducing loss and waste of these highly perishable produce items. It is well known that the colours of fruit and vegetables are often linked to the nutrients and phytochemicals they contain.
2. L-Citrulline Biochemistry
2.1. Endogenous Synthesis of L-Citrulline
De novo formation of CIT occurs in enterocytes [3], as described in
Figure 1. Hence, CIT concentration is currently considered to be a marker of intestinal function [10]. The substrates for intestinal CIT synthesis are amino acids derived from the diet, such as glutamine, proline and L-arginine (Arg) [11]. In enterocytes, part of the dietary and portal blood glutamine is catabolized to CIT by different enzymes (glutaminase, pyrroline-5-carboxylate synthase, ornithine aminotransferase (OAT), ornithine transcarbamylase (OTC) (also called ornithine carbamoyl transferase) and carbamoyl phosphate synthetase-I [12,13].
. Hence, CIT concentration is currently considered to be a marker of intestinal function [9]. The substrates for intestinal CIT synthesis are amino acids derived from the diet, such as glutamine, proline and L-arginine (Arg) [10]. In enterocytes, part of the dietary and portal blood glutamine is catabolized to CIT by different enzymes (glutaminase, pyrroline-5-carboxylate synthase, ornithine aminotransferase (OAT), ornithine transcarbamylase (OTC) (also called ornithine carbamoyl transferase) and carbamoyl phosphate synthetase-I [11][12].
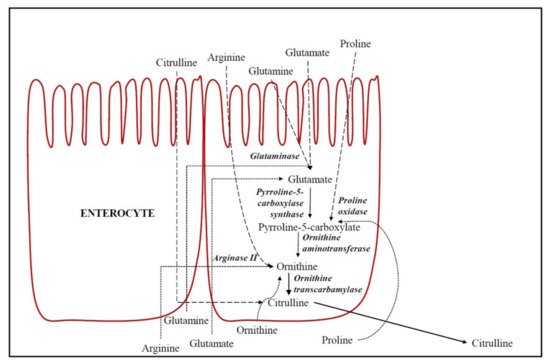
Figure 1.
De novo formation of citrulline in epithelial absorptive cells of the small intestine (enterocytes). Dashed lines indicate the amino acids flow from oral ingestion through enterocytes (apical transport) and dotted lines indicate the amino acids flow from portal blood through enterocytes (basolateral transport).
Glutaminase converts glutamine into glutamate and, therefore, the dietary and portal blood glutamate is catabolized in enterocytes to CIT in a common biosynthetic pathway to glutamine. However, the key regulation enzymes are pyrroline-5-carboxylate synthase and OAT, which are both unique to small intestinal enterocytes [11]. CIT accounts for 27.6% of metabolized glutamine [3]. Glutamine is generally considered the main precursor of intestinal CIT [11]. However, Marini et al. [14] demonstrated that dietary Arg was the principal precursor for CIT synthesis and that the proline contribution was low (3.4%), and glutamine was negligible (0.4%). Approximately 40% of dietary Arg is metabolized by arginase II into ornithine in enterocytes. Moreover, proline is also a precursor of CIT in the small intestine in enterocytes of postnatal pigs and healthy adults [15,16]. Proline is metabolized into P5C (Δ1-l-pyrroline-5-carboxylate) and subsequently into ornithine by proline oxidase and OAT, respectively [11]. Finally, the ornithine produced in enterocytes from these amino acids is metabolized into CIT by OTC. Ornithine may additionally be a direct CIT precursor from portal blood, above all when the supply of Arg is low [14]. OTC, in addition to catalyzing the conversion of ornithine into CIT, is a key enzyme in the urea cycle (
Glutaminase converts glutamine into glutamate and, therefore, the dietary and portal blood glutamate is catabolized in enterocytes to CIT in a common biosynthetic pathway to glutamine. However, the key regulation enzymes are pyrroline-5-carboxylate synthase and OAT, which are both unique to small intestinal enterocytes [10]. CIT accounts for 27.6% of metabolized glutamine [3]. Glutamine is generally considered the main precursor of intestinal CIT [10]. However, Marini et al. [13] demonstrated that dietary Arg was the principal precursor for CIT synthesis and that the proline contribution was low (3.4%), and glutamine was negligible (0.4%). Approximately 40% of dietary Arg is metabolized by arginase II into ornithine in enterocytes. Moreover, proline is also a precursor of CIT in the small intestine in enterocytes of postnatal pigs and healthy adults [14][15]. Proline is metabolized into P5C (Δ1-l-pyrroline-5-carboxylate) and subsequently into ornithine by proline oxidase and OAT, respectively [10]. Finally, the ornithine produced in enterocytes from these amino acids is metabolized into CIT by OTC. Ornithine may additionally be a direct CIT precursor from portal blood, above all when the supply of Arg is low [13]. OTC, in addition to catalyzing the conversion of ornithine into CIT, is a key enzyme in the urea cycle (
Figure 1). Therefore, the CIT circulating in the organism is the sum of CIT that we have absorbed from the diet foods plus the CIT production in the de novo synthesis in the intestine, with CIT being a biomarker of the functional small bowel enterocyte mass [10].
). Therefore, the CIT circulating in the organism is the sum of CIT that we have absorbed from the diet foods plus the CIT production in the de novo synthesis in the intestine, with CIT being a biomarker of the functional small bowel enterocyte mass [9].
2.2. L-Citrulline Metabolism
The metabolism of CIT, depending on the tissue distribution of the enzymes involved in it, follows three metabolic pathways (
Figure 2): Arg biosynthesis, the Arg-CIT-nitric oxide cycle, and the urea cycle [17]. There are three key enzymes, two involved in the CIT synthesis, nitric oxide synthase (NOS) and OTC, and one involved in CIT catabolism in mammals, argininosuccinate synthase (ASS) that converts CIT into argininosuccinate, which is used in both metabolic pathways [18].
): Arg biosynthesis, the Arg-CIT-nitric oxide cycle, and the urea cycle [16]. There are three key enzymes, two involved in the CIT synthesis, nitric oxide synthase (NOS) and OTC, and one involved in CIT catabolism in mammals, argininosuccinate synthase (ASS) that converts CIT into argininosuccinate, which is used in both metabolic pathways [17].
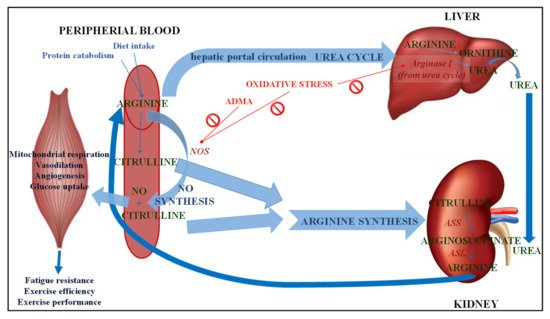
Figure 2.
Metabolism of citrulline. NO: Nitric oxide; NOS: Nitric oxide synthase; ADMA: Asymmetric dimethyl arginine; ASS: Argininosuccinate synthase; ASL: Argininosuccinate lyase.
2.2.1. Arginine
Arginine Biosynthesis
In mammals, Arg is classified as a semi-essential or conditionally essential amino acid, depending on the individual’s health status and stage of development [19]. Arg is a derivative from endogenous de novo Arg synthesis, body protein breakdown or dietary intake (
In mammals, Arg is classified as a semi-essential or conditionally essential amino acid, depending on the individual’s health status and stage of development [18]. Arg is a derivative from endogenous de novo Arg synthesis, body protein breakdown or dietary intake (
Figure 2). Arg synthesized from CIT represents 60% of the organism’s de novo Arg synthesis, although only 5–15% is circulating Arg [20]. Enterocytes release CIT into the portal circulation and transport it to the kidneys, where about 83% CIT catabolizes to Arg in the cells of proximal tubules [21]. ASS converts CIT into arginosuccinate in the presence of aspartate and ATP and arginosuccinate converts into fumarate and Arg by arginosuccinate lyase (ASL). Arg is synthesized in the intestinal-renal axis, where small intestine epithelial cells participate, along with the kidney proximal tubule cells [1]. Although other cells types and tissues contain the enzymes ASS and ASL to convert CIT to arginosuccinate and finally to Arg [22], the most important synthesis is in the liver and kidney. Moreover, elevated plasma concentrations of CIT can be a marker of kidney dysfunction, since plasma CIT is degraded in the kidney for the renal synthesis of Arg. Arg suffers intense hepatic catabolism, yet CIT is not metabolized by the liver, it seems that giving CIT supplements enables Arg to be administered whilst avoiding its hepatic uptake [3].
). Arg synthesized from CIT represents 60% of the organism’s de novo Arg synthesis, although only 5–15% is circulating Arg [19]. Enterocytes release CIT into the portal circulation and transport it to the kidneys, where about 83% CIT catabolizes to Arg in the cells of proximal tubules [20]. ASS converts CIT into arginosuccinate in the presence of aspartate and ATP and arginosuccinate converts into fumarate and Arg by arginosuccinate lyase (ASL). Arg is synthesized in the intestinal-renal axis, where small intestine epithelial cells participate, along with the kidney proximal tubule cells [1]. Although other cells types and tissues contain the enzymes ASS and ASL to convert CIT to arginosuccinate and finally to Arg [21], the most important synthesis is in the liver and kidney. Moreover, elevated plasma concentrations of CIT can be a marker of kidney dysfunction, since plasma CIT is degraded in the kidney for the renal synthesis of Arg. Arg suffers intense hepatic catabolism, yet CIT is not metabolized by the liver, it seems that giving CIT supplements enables Arg to be administered whilst avoiding its hepatic uptake [3].
Arginine Catabolism
Arg metabolism is highly compartmentalized. Arginase is a binuclear manganese metalloenzyme which catalyzes Arg hydrolysis to L-ornithine and urea (
). Two different genetic isoforms of arginase differ in their tissue distribution, subcellular localization, and immunological reactivity. Arginase I
is a cytosolic enzyme whose predominant expression lies in the liver, as part of the urea cycle, in which its function is the elimination of nitrogen generated in amino acid metabolism. However, to a much lesser extent, arginase I has been detected in extra-hepatic tissues such as endothelial cells as well as vascular smooth muscle cells [23]. On the other hand, arginase II is a mitochondrial enzyme that is widely distributed in extrahepatic cells and tissues (such as kidney, small intestine, brain, red blood cells and immune cells) [24]. Arginase II of the intestinal mucosa has a high activity, in fact the splanchnic area accounts for 40% of the dietary Arg extracted, thus explaining the low plasma concentrations of Arg. Therefore, the constitutive levels of arginase activity in endothelial cells limit the bioavailability of Arg as a substrate for the synthesis of endothelial nitric oxide (NO) by NOS and nitric oxide-dependent vasodilatory function [25]. Both enzymes, arginase and NOS, compete for the same substrate, and this competition depends on tissue type and the presence of stimuli [11].
a cytosolic enzyme whose predominant expression lies in the liver, as part of the urea cycle, in which its function is the elimination of nitrogen generated in amino acid metabolism. However, to a much lesser extent, arginase I has been detected in extra-hepatic tissues such as endothelial cells as well as vascular smooth muscle cells [22]. On the other hand, arginase II is a mitochondrial enzyme that is widely distributed in extrahepatic cells and tissues (such as kidney, small intestine, brain, red blood cells and immune cells) [23]. Arginase II of the intestinal mucosa has a high activity, in fact the splanchnic area accounts for 40% of the dietary Arg extracted, thus explaining the low plasma concentrations of Arg. Therefore, the constitutive levels of arginase activity in endothelial cells limit the bioavailability of Arg as a substrate for the synthesis of endothelial nitric oxide (NO) by NOS and nitric oxide-dependent vasodilatory function [24]. Both enzymes, arginase and NOS, compete for the same substrate, and this competition depends on tissue type and the presence of stimuli [10].
2.2.2. Nitric Oxide Biosynthesis
Nitric oxide (NO) can be synthesized endogenously in two ways: by a reduction of inorganic nitrate or through NOS enzyme action from Arg, producing CIT as a byproduct (
Figure 2) [26]. For this reaction to take place, oxygen must be present as well as reduced nicotinamide adenine dinucleotide phosphate (NADPH) as cosubstrates and the collaboration of cofactors flavin adenine dinucleotide (FAD), flavin mononucleotide (FMN), tetrahydrobiopterin and heme is required. ASS action can recycle the CIT generated as a byproduct to Arg, thereby increasing the Arg available for NO production [27]. There are three isoforms of NOS, located in different cells of the body: neuronal NOS (NOS1) which is present in neuronal cells, inducible NOS (NOS2) in macrophages and endothelial NOS (NOS3) in endothelial cells [1]. NOS2 is inducible, Ca
) [25]. For this reaction to take place, oxygen must be present as well as reduced nicotinamide adenine dinucleotide phosphate (NADPH) as cosubstrates and the collaboration of cofactors flavin adenine dinucleotide (FAD), flavin mononucleotide (FMN), tetrahydrobiopterin and heme is required. ASS action can recycle the CIT generated as a byproduct to Arg, thereby increasing the Arg available for NO production [26]. There are three isoforms of NOS, located in different cells of the body: neuronal NOS (NOS1) which is present in neuronal cells, inducible NOS (NOS2) in macrophages and endothelial NOS (NOS3) in endothelial cells [1]. NOS2 is inducible, Ca
2+
independent, and expressed by macrophages and other tissues during inflammation or when stimulated with bacterial endotoxins or inflammatory cytokines. NOS1 and NOS3 are constitutive enzymes regulated by intracellular calcium concentration and by the Ca
2+/calmodulin complex. Thus, NOS1 is involved in synaptic plasticity in the central nervous system (CNS), central regulation of blood pressure, smooth muscle relaxation, and vasodilatation via peripheral nitrergic nerves, which are involved in the relaxation of corpus cavernosum and penile erection [28]. NO generated from NOS1 acts as a neurotransmitter, while NO generated by NOS3 acts as a vasodilator. NOS3 maintains blood vessel dilation, blood pressure control, as well as several other vasoprotective and anti-atherosclerotic effects. Numerous cardiovascular risk factors cause oxidative stress, NOS3 uncoupling, and endothelial dysfunction in the vasculature. NO regulates vascular tone and blood flow by activating soluble guanylate cyclase (sGC) in the vascular smooth muscle. NO relaxes vascular smooth muscle by binding to the heme moiety of cytosolic guanylate cyclase, activating guanylate cyclase and increasing intracellular levels of cyclic guanosine 3′,5′-monophosphate, producing vasodilation [29].
/calmodulin complex. Thus, NOS1 is involved in synaptic plasticity in the central nervous system (CNS), central regulation of blood pressure, smooth muscle relaxation, and vasodilatation via peripheral nitrergic nerves, which are involved in the relaxation of corpus cavernosum and penile erection [27]. NO generated from NOS1 acts as a neurotransmitter, while NO generated by NOS3 acts as a vasodilator. NOS3 maintains blood vessel dilation, blood pressure control, as well as several other vasoprotective and anti-atherosclerotic effects. Numerous cardiovascular risk factors cause oxidative stress, NOS3 uncoupling, and endothelial dysfunction in the vasculature. NO regulates vascular tone and blood flow by activating soluble guanylate cyclase (sGC) in the vascular smooth muscle. NO relaxes vascular smooth muscle by binding to the heme moiety of cytosolic guanylate cyclase, activating guanylate cyclase and increasing intracellular levels of cyclic guanosine 3′,5′-monophosphate, producing vasodilation [28].
It also inhibits platelet aggregation and prevents the adhesion of leukocytes throughout the endothelial cell layer of blood vessels, inhibiting the proliferation of vascular smooth muscle cells by the inhibition of cytochrome oxidase, regulating the consumption of mitochondrial oxygen [30,31]. Abnormal vascular NO production and transport cause endothelial dysfunction with several cardiovascular pathologies such as hypertension, atherosclerosis and angiogenesis-associated disorders [19]. NO synthesis depends on Arg availability, which can be affected by: intracellular synthesis of Arg from CIT (which in turn depends on CIT being available), transport of extracellular Arg (cationic amino acid) and arginase activity (which competes with NOS for Arg). Additionally, asymmetric dimethylarginine (ADMA) is an endogenous inhibitor for all three NOS isoforms and has been reported as a risk factor for all causes of cardiovascular mortality [32].
It also inhibits platelet aggregation and prevents the adhesion of leukocytes throughout the endothelial cell layer of blood vessels, inhibiting the proliferation of vascular smooth muscle cells by the inhibition of cytochrome oxidase, regulating the consumption of mitochondrial oxygen [29][30]. Abnormal vascular NO production and transport cause endothelial dysfunction with several cardiovascular pathologies such as hypertension, atherosclerosis and angiogenesis-associated disorders [18]. NO synthesis depends on Arg availability, which can be affected by: intracellular synthesis of Arg from CIT (which in turn depends on CIT being available), transport of extracellular Arg (cationic amino acid) and arginase activity (which competes with NOS for Arg). Additionally, asymmetric dimethylarginine (ADMA) is an endogenous inhibitor for all three NOS isoforms and has been reported as a risk factor for all causes of cardiovascular mortality [31].
2.2.3. Urea Cycle
The urea cycle (
Figure 2), taking place in the liver, detoxifies ammonia from nitrogen from enteral sources (dietary protein) and muscle, thus metabolizing it into urea [3]. CIT metabolism in the liver consists of a highly compartmentalized metabolism, which is not connected with the other metabolic pathways that involve CIT [18]. Moreover, hepatocytes implicated in the urea cycle cannot take up CIT from the portal circulation (1). The two enzymes that control the first reactions of the urea cycle, carbamoyl phosphate synthetase (CPS) and OTC, are not exclusive to the liver but are also present in the small intestine. OTC joins carbamoyl phosphate and L-ornithine to produce CIT [33]. OTC deficiency could cause hypocitrullinemia, hyperammonemia and higher glutamine, alanine and lysine plasma concentrations, and even lead to coma or death [12]. However, this CIT pool is particularly labile: cytoplasmic ASS converts all synthesized CIT into argininosuccinate, and CIT is not released into general circulation [1,3]. Argininosuccinate is then transformed into Arg and fumarate by ASL. The cycle reaches its end when enzyme arginase metabolizes Arg into L-ornithine, thus liberating urea. Ornithine is mostly recycled while urea is synthesized, ultimately coming from de novo synthesis in the small intestine, although food sources may provide minor quantities. Finally, urea enters the kidneys to be excreted, thus completing the urea cycle, as a consequence of liver and kidney collaboration.
), taking place in the liver, detoxifies ammonia from nitrogen from enteral sources (dietary protein) and muscle, thus metabolizing it into urea [3]. CIT metabolism in the liver consists of a highly compartmentalized metabolism, which is not connected with the other metabolic pathways that involve CIT [17]. Moreover, hepatocytes implicated in the urea cycle cannot take up CIT from the portal circulation (1). The two enzymes that control the first reactions of the urea cycle, carbamoyl phosphate synthetase (CPS) and OTC, are not exclusive to the liver but are also present in the small intestine. OTC joins carbamoyl phosphate and L-ornithine to produce CIT [32]. OTC deficiency could cause hypocitrullinemia, hyperammonemia and higher glutamine, alanine and lysine plasma concentrations, and even lead to coma or death [11]. However, this CIT pool is particularly labile: cytoplasmic ASS converts all synthesized CIT into argininosuccinate, and CIT is not released into general circulation [1][3]. Argininosuccinate is then transformed into Arg and fumarate by ASL. The cycle reaches its end when enzyme arginase metabolizes Arg into L-ornithine, thus liberating urea. Ornithine is mostly recycled while urea is synthesized, ultimately coming from de novo synthesis in the small intestine, although food sources may provide minor quantities. Finally, urea enters the kidneys to be excreted, thus completing the urea cycle, as a consequence of liver and kidney collaboration.
3. L-Citrulline in Muscles
CIT supplementation in elderly malnourished rats increases muscle protein content by the stimulation of protein synthesis, but not of liver protein synthesis. This explains why whole-body protein synthesis does not change. On the other hand, neither protein mass nor protein synthesis are affected by CIT supplementation in the splanchnic area, thus confirming that that area is bypassed [18]. Additionally, branched-chain amino acids (BCAAs; leucine (LEU), valine, isoleucine), specifically LEU, are able to stimulate protein synthesis and decrease protein catabolism [34]. Le Plénier et al. [35], showed that in adult fasted rats, both LEU and CIT (despite having differing chemical structures and metabolisms) can stimulate muscle protein synthesis (MPS), but that LEU stimulates MPS in the post-prandial state and CIT in the post-absorptive state. Additionally, Faure et al. [36] demonstrated, through modeling protein-energy malnutrition in aged rats in the post-absorptive state that a CIT-enriched diet, increased muscle mass and muscle function was associated with improvement in both motor activity and maximal tetanic isometric force.
CIT supplementation in elderly malnourished rats increases muscle protein content by the stimulation of protein synthesis, but not of liver protein synthesis. This explains why whole-body protein synthesis does not change. On the other hand, neither protein mass nor protein synthesis are affected by CIT supplementation in the splanchnic area, thus confirming that that area is bypassed [17]. Additionally, branched-chain amino acids (BCAAs; leucine (LEU), valine, isoleucine), specifically LEU, are able to stimulate protein synthesis and decrease protein catabolism [33]. Le Plénier et al. [34], showed that in adult fasted rats, both LEU and CIT (despite having differing chemical structures and metabolisms) can stimulate muscle protein synthesis (MPS), but that LEU stimulates MPS in the post-prandial state and CIT in the post-absorptive state. Additionally, Faure et al. [35] demonstrated, through modeling protein-energy malnutrition in aged rats in the post-absorptive state that a CIT-enriched diet, increased muscle mass and muscle function was associated with improvement in both motor activity and maximal tetanic isometric force.
In humans, Jourdan et al. [37] were the first to demonstrate the muscle protein synthesis stimulated by CIT. In that study, increased muscle protein synthesis 25% was observed in healthy volunteers after three days on a relatively low-protein diet with CIT compared to a non-essential amino acid iso-nitrogenous mixture. This increased muscle protein synthesis appears to specifically affect CIT on muscle and is not an effect of increased nitrogen intake, as CIT did not affect whole-body protein synthesis [38]. This may be due to the specific effect of CIT on myofibrillar protein synthesis and expression in the muscle, which is the protein fraction involved in muscle contractility [39].
In humans, Jourdan et al. [36] were the first to demonstrate the muscle protein synthesis stimulated by CIT. In that study, increased muscle protein synthesis 25% was observed in healthy volunteers after three days on a relatively low-protein diet with CIT compared to a non-essential amino acid iso-nitrogenous mixture. This increased muscle protein synthesis appears to specifically affect CIT on muscle and is not an effect of increased nitrogen intake, as CIT did not affect whole-body protein synthesis [37]. This may be due to the specific effect of CIT on myofibrillar protein synthesis and expression in the muscle, which is the protein fraction involved in muscle contractility [38].
4. Pharmacokinetics and Pharmacodynamics of L-Citrulline
The characteristics of the pharmacokinetic parameters of CIT resemble those of related amino acids, Arg and ornithine, but not Cmax (peak concentration) which is several times greater in CIT than in Arg or ornithine [40]. Therefore, unlike Arg, the intestine or liver do not metabolize CIT; it is not a substrate for the arginase enzymes because CIT bypasses splanchnic extraction [18] and also inhibits its activity [41]. This could explain why plasma Arg levels rise rapidly and significantly when administered together, giving rise to the suggestion that Arg could pass through the gastrointestinal tract and liver without suffering the influence of intestinal and hepatic-first pass effects, which is likely to be due to CIT inhibiting arginase activity [30]. Oral CIT administration can be used to enhance systemic CIT and Arg availability; it is bioavailable and losses in urine are very low [42]. In a combined sample of middle-aged women and men, Schwedhelm et al. [43] found that oral CIT supplementation, at a dose of 1.5 g twice daily for one week, increased the plasma concentrations of CIT and Arg in a dose-dependent manner and augmented plasma Arg levels with greater effectiveness than by supplementing Arg in healthy subjects. Moinard et al. [44] found a similar behavior in healthy elderly subjects when a single dosing caused a marked increase in Arg availability when CIT (10 g) was administered, compared with Arg (9.94 g) administration itself. Previously, in a study performed in healthy men, using oral loads (2, 5, 10, or 15 g CIT) administered at random, Moinard et al. [40], had found that the plasma CIT concentration increased rapidly and massively (10-fold at the 2 g load to 100-fold at the 15 g load) and returned to baseline values within 5–8 h post-loading. These authors demonstrated that CIT was well tolerated (no side effects) and did not induce gastrointestinal disorders at a high dose (i.e., 15 g), thus suggesting that intestinal absorption of CIT is not a limiting step. However, Arg production was lower than expected when 15 g of CIT was administered. This could be explained by the decreased renal conversion of CIT to Arg and/or, a possible saturation of its transporters a certain dose of CIT. Several authors have proposed that CIT uptake uses a different transport system to Arg which is mainly transported through Na+-independent cationic amino acid transporters (CAT-1, 2 and 3) [45]. CIT is transported across the enterocytes to the portal circulation, likely using the Na+-dependent, neutral amino acid, including the ASC or B
The characteristics of the pharmacokinetic parameters of CIT resemble those of related amino acids, Arg and ornithine, but not Cmax (peak concentration) which is several times greater in CIT than in Arg or ornithine [39]. Therefore, unlike Arg, the intestine or liver do not metabolize CIT; it is not a substrate for the arginase enzymes because CIT bypasses splanchnic extraction [17] and also inhibits its activity [40]. This could explain why plasma Arg levels rise rapidly and significantly when administered together, giving rise to the suggestion that Arg could pass through the gastrointestinal tract and liver without suffering the influence of intestinal and hepatic-first pass effects, which is likely to be due to CIT inhibiting arginase activity [29]. Oral CIT administration can be used to enhance systemic CIT and Arg availability; it is bioavailable and losses in urine are very low [41]. In a combined sample of middle-aged women and men, Schwedhelm et al. [42] found that oral CIT supplementation, at a dose of 1.5 g twice daily for one week, increased the plasma concentrations of CIT and Arg in a dose-dependent manner and augmented plasma Arg levels with greater effectiveness than by supplementing Arg in healthy subjects. Moinard et al. [43] found a similar behavior in healthy elderly subjects when a single dosing caused a marked increase in Arg availability when CIT (10 g) was administered, compared with Arg (9.94 g) administration itself. Previously, in a study performed in healthy men, using oral loads (2, 5, 10, or 15 g CIT) administered at random, Moinard et al. [39], had found that the plasma CIT concentration increased rapidly and massively (10-fold at the 2 g load to 100-fold at the 15 g load) and returned to baseline values within 5–8 h post-loading. These authors demonstrated that CIT was well tolerated (no side effects) and did not induce gastrointestinal disorders at a high dose (i.e., 15 g), thus suggesting that intestinal absorption of CIT is not a limiting step. However, Arg production was lower than expected when 15 g of CIT was administered. This could be explained by the decreased renal conversion of CIT to Arg and/or, a possible saturation of its transporters a certain dose of CIT. Several authors have proposed that CIT uptake uses a different transport system to Arg which is mainly transported through Na+-independent cationic amino acid transporters (CAT-1, 2 and 3) [44]. CIT is transported across the enterocytes to the portal circulation, likely using the Na+-dependent, neutral amino acid, including the ASC or B
0,+-amino acid transporters located in those cells [13,45,46,47].
-amino acid transporters located in those cells [12][44][45][46].
According to the pharmacodynamic CIT parameters, this amino acid is well tolerated and under short-term application, no side effects such as gastrointestinal disorders were observed, even when high doses (i.e., 15 g) were applied. This is a positive feature of CIT, since in previous studies the administration of related amino acids (>9 g/day of Arg for 1 week or >10 g/day of ornithine in a single dose) caused nausea, vomiting or diarrhea [48]. The explanation for this may lie in the rapid saturation of intestinal absorption of ornithine and Arg, with high loads inducing osmotic diarrhea [40]. In contrast to Arg and ornithine, intestinal CIT absorption is not a limiting factor in CIT bioavailability, since CIT is well tolerated and recognized as safe for oral consumption [18,49]. The highest doses of CIT reported are 0.18 g/kg/day (approximately 12.6 g/day in a person of 70 kg) for 7 days [50] and a single dose of 15 g [40].
According to the pharmacodynamic CIT parameters, this amino acid is well tolerated and under short-term application, no side effects such as gastrointestinal disorders were observed, even when high doses (i.e., 15 g) were applied. This is a positive feature of CIT, since in previous studies the administration of related amino acids (>9 g/day of Arg for 1 week or >10 g/day of ornithine in a single dose) caused nausea, vomiting or diarrhea [47]. The explanation for this may lie in the rapid saturation of intestinal absorption of ornithine and Arg, with high loads inducing osmotic diarrhea [39]. In contrast to Arg and ornithine, intestinal CIT absorption is not a limiting factor in CIT bioavailability, since CIT is well tolerated and recognized as safe for oral consumption [17][48]. The highest doses of CIT reported are 0.18 g/kg/day (approximately 12.6 g/day in a person of 70 kg) for 7 days [49] and a single dose of 15 g [39].
References
- Bescós, R.; Sureda, A.; Tur, J.A.; Pons, A. The Effect of Nitric-Oxide-Related Supplements on Human Performance. Sports Med. 2012, 42, 99–117.
- Mandel, H.; Levy, N.; Izkovitch, S.; Korman, S.H. Elevated plasma citrulline and arginine due to consumption of Citrullus vulgaris (watermelon). J. Inherit. Metab. Dis. 2005, 28, 467–472.
- Windmueller, H.G.; Spaeth, A.E. Source and fate of circulating citrulline. Am. J. Physiol. Endocrinol. Metab. 1981, 241, E473–E480.
- Rimando, A.M.; Perkins-Veazie, P.M. Determination of citrulline in watermelon rind. J. Chromatogr. A 2005, 1078, 196–200.
- Tarazona-Díaz, M.P.; Viegas, J.; Moldao Martins, M.; Aguayo, E. Bio-active compounds of different cultivars from flesh and by-product of fresh-cut watermelons. J. Sci. Food Agric. 2011, 91, 805–812.
- Joshi, V.; Joshi, M.; Silwal, D.; Noonan, K.; Rodriguez, S.; Penalosa, A. Systematized biosynthesis and catabolism regulate citrulline accumulation in watermelon. Phytochemistry 2019, 162, 129–140.
- Aguayo, E.; Tarazona-Díaz, M.P. National Patent: Procedimiento Para la Obtención de un Extracto de L-Citrulina a Partir de Plantas Cucurbitáceas. ES2394250 A1. Available online: (accessed on 12 November 2020).
- FAOSTAT Agriculture Data. 2021. Available online: (accessed on 12 November 2020).
- Crenn, P.; Messing, B.; Cynober, L. Citrulline as a biomarker of intestinal failure due to enterocyte mass reduction. Clin. Nutr. 2008, 27, 328–339.
- Wu, G.; Morris, S.M., Jr. Arginine metabolism: Nitric oxide and beyond. Biochem. J. 1998, 336, 1–17.
- Breuillard, C.; Cynober, L.; Moinard, C.; Crenn, P.; Curis, E.; Chaumeil, J.C.; Cynober, L.; Sfar, S. Citrulline and nitrogen homeostasis: An overview. Amino Acids 2015, 47, 685–691.
- Bahri, S.; Zerrouk, N.; Aussel, C.; Moinard, C.; Crenn, P.; Curis, E.; Chaumeil, J.C.; Cynober, L.; Sfar, S. Citrulline: From metabolism to therapeutic use. Nutrition 2013, 29, 479–484.
- Marini, J.C.; Didelija, I.C.; Castillo, L.; Lee, B. Plasma Arginine and Ornithine Are the Main Citrulline Precursors in Mice Infused with Arginine-Free Diets. J. Nutr. 2010, 140, 1432–1437.
- Tomlinson, C.; Rafii, M.; Ball, R.O.; Pencharz, P.B. Arginine Can Be Synthesized from Enteral Proline in Healthy Adult Humans. J. Nutr. 2011, 141, 1432–1436.
- Wu, G. Intestinal Mucosal Amino Acid Catabolism. J. Nutr. 1998, 128, 1249–1252.
- Barzał, J.A.; Szczylik, C.; Rzepecki, P.; Jaworska, M.; Anuszewska, E. Plasma citrulline level as a biomarker for cancer therapy-induced small bowel mucosal damage. Acta Biochim. Pol. 2014, 61, 615–631.
- Curis, E.; Nicolis, I.; Moinard, C.; Osowska, S.; Zerrouk, N.; Bénazeth, S.; Cynober, L. Almost all about citrulline in mammals. Amino Acids 2005, 29, 177–205.
- Luiking, Y.C.; Ten Have, G.A.M.; Wolfe, R.R.; Deutz, N.E.P. Arginine de novo and nitric oxide production in disease states. Am. J. Physiol.-Endocrinol. Metab. 2012, 303, E1177–E1189.
- Morris, S.M. Regulation of arginine availability and its impact on NO synthesis. In Nitric Oxide: Biology and Pathobiology; Academic Press: Cambridge, MA, USA, 2020; pp. 187–197.
- Van De Poll, M.C.G.; Soeters, P.B.; Deutz, N.E.P.; Fearon, K.C.H.; DeJong, C.H.C. Renal metabolism of amino acids: Its role in interorgan amino acid exchange. Am. J. Clin. Nutr. 2004, 79, 185–197.
- Mori, M.; Gotoh, T. Arginine Metabolic Enzymes, Nitric Oxide and Infection. J. Nutr. 2004, 134, 2820S–2825S; discussion 2853S.
- Durante, W.; Johnson, F.K.; Johnson, R.A. Arginase: A Critical Regulator of Nitric Oxide Synthesis and Vascular Function. Clin. Exp. Pharmacol. Physiol. 2007, 34, 906–911.
- Pernow, J.; Jung, C. Arginase as a potential target in the treatment of cardiovascular disease: Reversal of arginine steal? Cardiovasc. Res. 2013, 98, 334–343.
- Vanhoutte, P.M.; Shimokawa, H.; Feletou, M.; Tang, E.H.C. Endothelial dysfunction and vascular disease-A 30th anniversary update. Acta Physiol. 2017, 219, 22–96.
- Bode-Böger, S.M.; Böger, R.H.; Galland, A.; Tsikas, D.; Frölich, J.C. L-arginine-induced vasodilation in healthy humans: Pharmacokinetic-pharmacodynamic relationship. Br. J. Clin. Pharmacol. 1998, 46, 489–497.
- Husson, A.; Brasse-Lagnel, C.; Fairand, A.; Renouf, S.; Lavoinne, A. Argininosuccinate synthetase from the urea cycle to the citrulline-NO cycle. Eur. J. Biochem.. 2003, 270, 1887–1899.
- Foörstermann, U.; Sessa, W.C. Nitric oxide synthases: Regulation and function. Eur. Heart J. 2012, 33, 829–837.
- Suzuki, T.; Morita, M.; Kobayashi, Y.; Kamimura, A. Oral L-citrulline supplementation enhances cycling time trial performance in healthy trained men: Double-blind randomized placebo-controlled 2-way crossover study. J. Int. Soc. Sports Nutr. 2016, 13, 6.
- Morita, M.; Hayashi, T.; Ochiai, M.; Maeda, M.; Yamaguchi, T.; Ina, K.; Kuzuya, M. Oral supplementation with a combination of L-citrulline and L-arginine rapidly increases plasma L-arginine concentration and enhances NO bioavailability. Biochem. Biophys. Res. Commun. 2014, 454, 53–57.
- Luiking, Y.C.; Engelen, M.P.; Deutz, N.E. Regulation of nitric oxide production in health and disease. Curr. Opin. Clin. Nutr. Metab. Care 2010, 13, 97–104.
- Böger, R.H.; Sullivan, L.M.; Schwedhelm, E.; Wang, T.J.; Maas, R.; Benjamin, E.J.; Schulze, F.; Xanthakis, V.; Benndorf, R.A.; Vasan, R.S. Plasma Asymmetric Dimethylarginine and Incidence of Cardiovascular Disease and Death in the Community. Circulation 2009, 119, 1592–1600.
- Kaore, S.N.; Amane, H.S.; Kaore, N.M. Citrulline: Pharmacological perspectives and its role as an emerging biomarker in future. Fundam. Clin. Pharmacol. 2013, 27, 35–50.
- Cynober, L.; de Bandt, J.P.; Moinard, C. Leucine and citrulline: Two major regulators of protein turnover. In Nutrition in Intensive Care Medicine: Beyond Physiology; Singer, P., Ed.; Karger Medical and Scientific Publishers: Basel, Switzerland, 2012; Volume 105, pp. 97–105.
- Le Plénier, S.; Walrand, S.; Noirt, R.; Cynober, L.; Moinard, C. Effects of leucine and citrulline versus non-essential amino acids on muscle protein synthesis in fasted rat: A common activation pathway? Amino Acids 2012, 43, 1171–1178.
- Faure, C.; Raynaud-Simon, A.; Ferry, A.; Daugé, V.; Cynober, L.; Aussel, C.; Moinard, C. Leucine and citrulline modulate muscle function in malnourished aged rats. Amino Acids 2011, 42, 1425–1433.
- Jourdan, M.; Nair, K.S.; Carter, R.E.; Schimke, J.; Ford, G.C.; Marc, J.; Aussel, C.; Cynober, L. Citrulline stimulates muscle protein synthesis in the post-absorptive state in healthy people fed a low-protein diet–A pilot study. Clin. Nutr. 2015, 34, 449–456.
- Goron, A.; Lamarche, F.; Cunin, V.; Dubouchaud, H.; Hourdé, C.; Noirez, P.; Corne, C.; Couturier, K.; Sève, M.; Fontaine, E.; et al. Synergistic effects of citrulline supplementation and exercise on performance in male rats: Evidence for implication of protein and energy metabolisms. Clin. Sci. 2017, 131, 775–790.
- Ventura, G.; Noirez, P.; Breuillé, D.; Godin, J.P.; Pinaud, S.; Cleroux, M.; Choisy, C.; Le Plénier, S.; Bastic, V.; Neveux, N.; et al. Effect of citrulline on muscle functions during moderate dietary restriction in healthy adult rats. Amino Acids 2013, 45, 1123–1131.
- Moinard, C.; Nicolis, I.; Neveux, N.; Darquy, S.; Benazeth, S.; Cynober, L. Dose-ranging effects of citrulline administration on plasma amino acids and hormonal patterns in healthy subjects: The Citrudose pharmacokinetic study. Br. J. Nutr. 2008, 99, 855–862.
- Romero, M.J.; Platt, D.H.; Caldwell, R.W. Therapeutic Use of Citrulline in Cardiovascular Disease. Cardiovasc. Drug Rev. 2006, 24, 275–290.
- Rougé, C.; Des Robert, C.; Robins, A.; Le Bacquer, O.; Volteau, C.; De La Cochetière, M.F.; Darmaun, D. Manipulation of citrulline availability in humans. Am. J. Physiol. Gastrointest. Liver Physiol. 2007, 293, 1061–1067.
- Schwedhelm, E.; Maas, R.; Freese, R.; Jung, D.; Lukacs, Z.; Jambrecina, A.; Spickler, W.; Schulze, F.; Böger, R.H. Pharmacokinetic and pharmacodynamic properties of oral L-citrulline and L-arginine: Impact on nitric oxide metabolism. Br. J. Clin. Pharmacol. 2008, 65, 51–59.
- Moinard, C.; Maccario, J.; Walrand, S.; Lasserre, V.; Marc, J.; Boirie, Y.; Cynober, L. Arginine behaviour after arginine or citrulline administration in older subjects. Br. J. Nutr. 2016, 115, 399–404.
- Closs, E.I.; Simon, A.; Vékony, N.; Rotmann, A. Plasma Membrane Transporters for Arginine. J. Nutr. 2004, 134, 2752S–2759S.
- Vadgama, J.V.; Evered, D.F. Characteristics of L-Citrulline Transport across Rat Small Intestine In Vitro. Pediatr. Res. 1992, 32, 472–478.
- Allerton, T.D.; Proctor, D.N.; Stephens, J.M.; Dugas, T.R.; Spielmann, G.; Irving, B.A. L-Citrulline Supplementation: Impact on Cardiometabolic Health. Nutrients 2018, 10, 921.
- Grimble, G.K. Adverse Gastrointestinal Effects of Arginine and Related Amino Acids. J. Nutr. 2007, 137, 1693S–1701S.
- Collins, J.K.; Wu, G.; Perkins-Veazie, P.; Spears, K.; Claypool, P.L.; Baker, R.A.; Clevidence, B.A. Watermelon consumption increases plasma arginine concentrations in adults. Nutrients 2007, 23, 261–266.
- Thibault, R.; Flet, L.; Vavasseur, F.; Lemerle, M.; Ferchaud-Roucher, V.; Picot, D.; Darmaun, D. Oral citrulline does not affect whole body protein metabolism in healthy human volunteers: Results of a prospective, randomized, double-blind, cross-over study. Clin. Nutr. 2011, 30, 807–811.
