Heart failure (HF) remains a leading cause of morbidity, hospitalization, and mortality worldwide. Advancement of mechanical circulatory support technology has led to the use of continuous-flow left ventricular assist devices (LVADs), reducing hospitalizations, and improving quality of life and outcomes in advanced HF. Recent studies have highlighted how metabolic and endocrine dysfunction may be a consequence of, or associated with, HF, and may represent a novel (still neglected) therapeutic target in the treatment of HF. On the other hand, it is not clear whether LVAD support, may impact the outcome by also improving organ perfusion as well as improving the neuro-hormonal state of the patients, reducing the endocrine dysfunction. Moreover, endocrine function is likely a major determinant of human homeostasis, and is a key issue in the recovery from critical illness. Care of the endocrine function may contribute to improving cardiac contractility, immune function, as well as infection control, and rehabilitation during and after a LVAD placement.
1. Thyroid Dysfunction
Thyroid hormone (TH) signaling is a relevant component of the adaptive response of the myocardium to stress, and plays a critical role in regulating both heart rate and contractility of myocytes: hyperthyroidism is correlated with atrial arrhythmias, hypertension, and heart failure, and increases the risk of heart failure and mortality in cardiopathic patients
[1][13]. As in the majority of hormones, TH is regulated by a number of pathways, and its production and endocrine release is impaired by the continuous flow determined by VADs.
The thyroid gland secretes T3 (triiodothyronine) and T4 (levothyroxine) under stimulation by TSH, the pituitary synthesis of which is downregulated with feedback loop mechanism by T3 and T4, and upregulated from hypothalamic synthesis of thyrotropin releasing hormone (TRH). The thyroid gland primarily secretes T4, which is then de-iodinated in its active form, T3, in the liver, kidney, and skeletal muscle.
1.1. Effects of Thyroid Hormones on Cardiac Function
The effect determined by TH on myocyte contractility is due to both genomic and non-genomic mechanisms. T3 binds thyroid hormones receptors (TRs) on the cellular membrane, promoting transcription processes involved in the synthesis of proteins responsible for cardiac contractility. For what concerns non-genomic activities, T3 can induce changes in the myocyte membrane, involving changes in sodium, potassium, and calcium ion channels. Both mechanisms lead to upregulation of sarcoplasmic reticulum calcium-activated ATPase.
In summary, TH acts on the cardiac function in the following ways ():
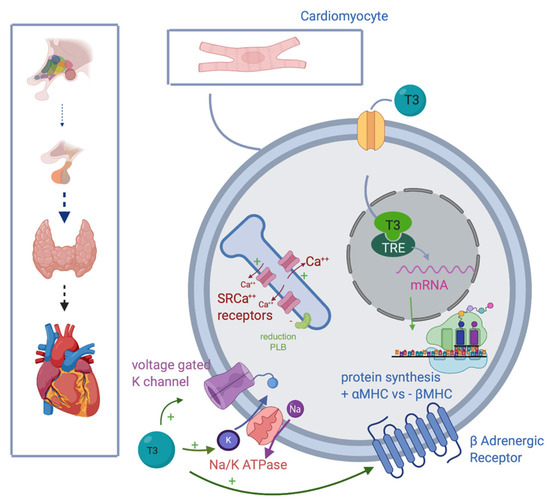
Figure 1. T3 improves myocytes and heart contractility, inducing intranuclear mRNA transcription, improving synthesis of fast αMHC, and reducing one of the slow βMHC; stimulating transmembrane expression of voltage-gated K channel, Na/K ATPase and βadrenergic receptors; upregulating SRCa++ rec, while inhibiting PLB. Abbreviations: T3, triiodothyronine; mRNA, messenger RNA; αMHC, α myosin heavy chain; Na/K ATPase, sodium-potassium adenosine triphosphatase; SRCa++ receptors, sarcoendoplasmic reticulum (SR) Ca(2+) receptors; PLB, phospholambane; TRE, thyroid hormones response element; βMHC, β myosin heavy chain.
- -
-
Promoting synthesis of the α isoforms of the myosin heavy chain (faster contractility), and reducing β isoform (slow contractility), enhancing systolic function.
- -
-
Upregulating β1 receptors, improving adrenergic responsiveness.
- -
-
Upregulating Na/K ATPase and voltage gating potassium channel
[2][14].
- -
-
Inducing sarcoendoplasmic reticulum (SR) Ca(2+) receptors SERCA2a and down-regulation of phospholamban ATPase.
- -
-
Acting on the sinoatrial node, T3 can also improve heart rate.
On the other hand, transcription is inhibited in the absence of T3, inducing depression of myocardial function
[1][2][13,14]. Moreover, acting on vascular smooth muscle cells (VSM), T3 decreases vascular resistance, improving arterial compliance, with a direct effect on VSM, and stimulating production of nitric oxide, with a 50% cardiac output increase
[3][15]. Hypothyroidism is characterized by the opposite scenario: increased resistance and reduction of cardiac output and contractility. Moreover, heart failure can be related to altered conversion of T4 to T3.
1.2. Considerations after LVAD Implant and Potential Implications for Supplementation
VAD determines prolonged cardiac unloading, leading to molecular and structural modifications in heart cells. According to Ito et al., unloading is responsible for impaired Ca
++ homeostasis, switch from α to β MHC (myocardial heavy chains) protein synthesis, and suppression of SERCA function. All of these parameters improve after T3 administration, and several studies have shown that treatment with T3 could hinder impaired Ca
++ homeostasis and the shift from α to β MHC, improving cardiac contractility. Moreover, T3 is responsible for the restoration of cardiac activity contrasting PLB (phospholambane) phosphorylation suppression induced by VAD. ATPase function is also suppressed, and improves after treatment with T3
[4][16].
The benefits induced by T3 treatments are stressed in a case report described by Letsou in 2013 concerning the insurgence of thyrotoxicosis in a young patient affected with CHF on VAD. They observed the resolution of heart failure after an episode of thyrotoxicosis, and stressed the improvement in contractility function to the high level of TH. Once THs went back to their normal levels, the pathology seemed to be resolved
[5][17]. In another study, Dipla et al. investigated the role of LVADs in improving myocyte contractility and their responsiveness to isoprenaline. Their findings highlighted the positive response of the myocytes, attributing the contractile improvements to changes in Ca
++ homeostasis and SRCa ATPase activity. Furthermore, β responsiveness to isoprenaline was investigated in one study. Isoprenaline upregulated the expression of β receptors, already downregulated by the long-term exposure to sympathetic agonists in HF patients, thus allowing a reduction in inotropic drug administration. Moreover, changes in PLB homeostasis were hypothesized
[6][18].
Afterload reduction and increasing of myocyte contractility are essential elements in the therapy of HF
[6][18]. TH can change myocyte contractility, modifying Ca
++ homeostasis, SRCa, ATPase, upregulating β receptors, and stimulating NO secretion from smooth muscle cells. They could conceivably represent a possible resource in CHF treatment. Or, at least, thyroid hormone homeostasis should be considered central in patients with CHF and VAD.
CHF pattern is characterized by an altered pathway of synthesis of myocyte heavy chains (MHC), and this has been linked to modification in thyroid hormones axis; more precisely to a different pathway of TR (thyroid receptors) as a stress response to adrenergic stimuli
[7][8][19,20].
Current therapies for HF are based on optimizing cardiocirculatory hemodynamics without focusing on myocyte remodeling. Pantos et al. illustrated the role of T3 in restoring myocyte contractility, noticing that low T3, known as low T3 syndrome, is considered a risk factor for HF and increased mortality, while a spontaneous recovery of T3 level is evidence of a better prognosis. Moreover, TH has been used after heart transplantation in order to improve the prognosis. Since the principle use of T4 instead of T3 led to inconsistent results, the real benefits have to be confirmed
[9][21]. In any event, given the complex correlations between different factors in VAD carriers, TH may not be considered merely a bystander and a signal of general improvement, but every minimal part of the homeostatic restoration should be considered during recovery
[1][3][13,15].
On the other hand, hyperthyroidism is associated with tachyarrhythmias and pulmonary hypertension, both detrimental after VAD placement, even more when associated with chronic myocardial ischemia. Thus, it is important to monitor and maintain the correct plasma level of TH.
Nguyen et al. found that in their cohort of patients affected with thyroid impairment, an improvement of their metabolic status was observed after implantation of VAD
[10][6]. These studies suggest the possibility that T3 therapy could improve patient response to VAD placement, sustaining cardiac contractility and reducing vascular resistance.
Thyroid function in LVAD carriers and with HF likely still needs to be elucidated, but currently a reasonable balance between risks and benefits impose that attention should be given to TH measurements, and supplementation should be started early in hypothyroidism, as well as in the case of normal free fractions of the hormones with high TSH levels. This approach is not derived from studies on LVAD patients or HF patients, but is a common value for critically ill patients. The relevant topic is already a constant and protocolized monitoring to detect early potential dysfunction.
2. Vitamin D Deficiency and Supplementation
Vitamin D is a pre-hormone that has a key function in bone health and calcium metabolism. Across the last decade, this molecule has seen renewed attention by the healthcare community in several fields, since its receptor has been found in almost all cells and tissues of the human body, and several endocrine paracrine and autocrine functions have been elucidated. Moreover, currently, at a population level, vitamin D deficiency is diffused worldwide in a pandemic fashion for reasons still unclear, but likely linked to changes in the consumption of food, life-style attitudes, obesity, ageing, and high prevalence of chronic cardiovascular and immunity-related diseases
[11][22]. Lower vitamin D levels are associated with cardiac, pulmonary, and metabolic diseases, as well as with general morbidity and mortality. An important role for vitamin D is clearly defined for regulating immunity, either in modulation in the case of autoimmune diseases, or increasing the power of innate immunity for prevention and response to viral and bacterial infections
[12][13][14][23,24,25]. This association is even more relevant in critically ill patients, where vitamin D can be considered a real pro-survival hormone
[15][26]. But, despite much interesting data and a strong pathophysiological scenario, evidence from standard randomized controlled trials is still far from conclusive
[16][17][27,28].
23.1. Effects on Cardiac Function
In the cases of the heart dysfunction, vitamin D seems to be a contributing factor in optimizing cardiac function
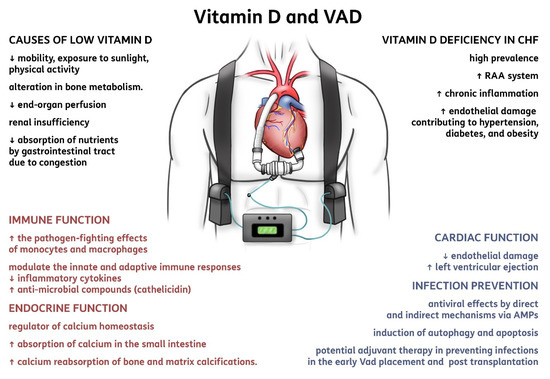
[18][29] (). First, in patients affected with CHF, vitamin D deficiency has a high prevalence; it may activate the renin-angiotensin-aldosterone system, favor chronic inflammation, and cause endothelial damage that is implicated as contributing to hypertension, diabetes, and obesity
[19][30]. By definition, patients with advanced HF and congestive signs have reduced mobility, exposure to sunlight, and physical activity, with a consequent alteration in bone and vitamin D metabolism
[20][31]. A reduced absorption of nutrients due to intestinal congestion is relevant, and the use of diuretics and hyperaldosteronism can also play a role, with relevant losses of Ca and Mg, as well as the frequent association with diabetes and variable severity of concomitant renal insufficiency. The increased risk of osteoporosis and fractures is clear in such group of patients, but the restoration of adequate bone and muscle metabolism is paramount for the general improvement of patients with cardiac surgery, CHF, heart transplantation, and LVAD placement
[21][32]. Wu et al. have highlighted that patients with HF have a high prevalence of severe bone remodeling (namely resorption), which has a link to mortality
[22][33].
Figure 2. Summary of vitamin D implications in ventricular assist devices. Abbreviations: CHF, chronic heart failure; RAA system, renin-angiotensin-aldosterone system; VAD, ventricular assist device; AMPs, anti-microbial peptides.
Guidelines on Vitamin D defined 50 nmol/L as “vitamin D requirement of nearly all normal healthy persons,” using bone health as the principal basis
[23][24][34,35]. Similarly, critically ill patients display a very high prevalence of vitamin D deficiency, clearly associated with greater illness severity, morbidity, and mortality in both adult and pediatric intensive care unit (ICU) patients, as well as medical and surgical intensive care units
[25][36]. In LVAD carriers, the concepts of metabolic support verified in critically ill and CHF patients fit very well. Also in this setting, it should still be determined whether low vitamin D is an innocent bystander, simply reflecting greater disease severity, or represents an independent and modifiable risk factor amenable to normalization through supplementation
[26][27][37,38]. The question is meaningful since in LVAD implants many factors contribute to low levels: hemodilution, frequent renal failure, reduced production and conversion by the liver, altered mineral metabolism, reduced synthesis of vitamin D binding protein, higher consumption during the acute phase of disease and systemic inflammation, and increased tissue demand and enhanced catabolism of metabolites
[28][39]. More data are emerging from basic science about immediate and late effects of vitamin D supplementation on endocrine, autocrine and paracrine, and genomic targets; it is likely that the mere monitoring of vitamin D supplementation based on bone turnover markers is not adequate for this purpose
[29][40]. Moreover, in a setting of chronic patients, with a number of repeated confounding factors and concomitant risk factors, the use of mortality or the number of mechanical circulatory support as clinical outcomes, should be considered with caution when the effect of the vitamin D supplementation is tested
[29][40]. However, differently from HF patients, LVAD was able to determine a transient effect of the FGF-23 (effector of vitamin D metabolism, but also linked to physical activity and kidney function), and to increase significantly the level of circulating vitamin D
[28][39].
A specific effect of vitamin D on the immunity and prevention (and possibly also treatment) of infections has been advocated, and that may be critical for CHF patients with VAD, and when they proceed to heart transplantation
[25][30][36,41]. Given the inflammation and immunosuppression in the setting of transplantation, any adjuvant treatment should be considered to reduce the impact of infections in the early VAD placement or transplantation for the single patient, and from a wide healthcare system view, since such patients when they experience an infection it is often a complicated one and may impair resource availability and the clinical outcome of the patient
[31][32][33][42,43,44].
23.2. Considerations after LVAD Implant and Potential Implications for Supplementation
In intensive care, usually loading dose (followed by a daily dose) is necessary to improve vitamin D levels rapidly
[34][45]. There is no consensus on the optimal level for vitamin D supplementation, ranging from 400 to 2000 IU daily
[35][46]. A safe and commonly available dose of 25 μg vitamin D3 (1000 IU) raises 25-hydroxyvitamin D (25(OH)D) serum level by 15–25 nmol/L on average (over weeks/months)
[35][36][37][38][46,47,48,49]. The toxicity is also variable; in fact, the upper daily limit suggested by the Endocrine Society is 10,000 IU
[23][34], while the Institute of Medicine (USA) and The European Food and Safety Authority recommend staying below 4000 IU/day (100 µg)
[39][40][50,51]. In any event, in clinical practice rarely are toxic levels of vitamin D reached, and the patients remain, most of the time, vitamin D deficient. Moreover, the availability of vitamin D supplementation still varies from hospital to hospital, and the two most frequent molecules available are cholecalciferol (needs conversion by the liver enzymes) and calcifediol (bypasses liver activity but still needs last activation). Awaiting specific evidence it is reasonable to adopt the already available recommendations for supplementation in critically ill patients according to the recent nutrition guidelines released by ESPEN.
32. Erythropoiesis-Stimulating Agents and Iron Supplementation
Numerous mechanisms beyond low cardiac output sustain impaired exercise tolerance in patients with chronic heart failure. Among them, anemia is well recognized
[41][42][43][52,53,54]. In patients with HFrEF, anemia has multiple effects: on the one hand, it increases myocardial work in the attempt to augment oxygen delivery; on the other, increasing the Hb level with erythropoiesis stimulating agents (ESAs) decreases left ventricular ejection fraction (LVEF) and cardiac output, probably because of increased blood viscosity and decreased nitric oxide availability
[44][45][46][55,56,57].
Anemia is very common in patients with heart failure (HF), affecting 50% of patients with acute decompensated HF
[47][48][49][58,59,60]. Moreover, it is associated with mortality with a cut-off value of 12.0 g per deciliter
[50][61]. Therefore, the hemoglobin level might simply be a marker of poor prognosis in heart failure rather than a therapeutic target.
The pathogenesis of anemia in HF is multifactorial, but iron deficiency (ID) is extremely common
[43][54], with a reported prevalence of between 30 and 70%
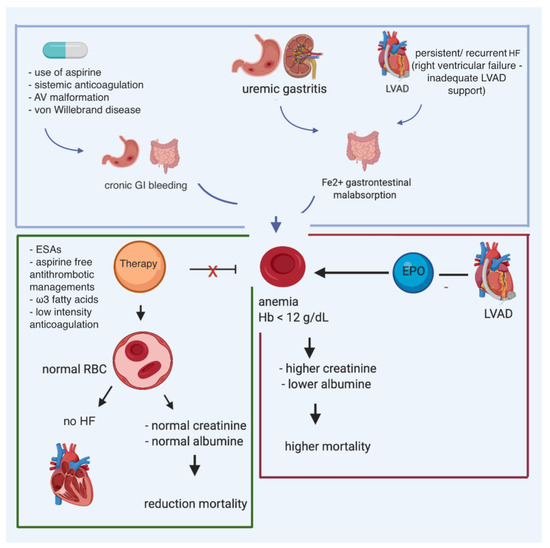
[50][51][61,62]. Patients with severe anemia often have features of worse HF, with more extensive left ventricular (LV) remodeling and higher levels of biomarkers of advanced HF, higher inflammatory and collagen markers, and worse renal function. These factors lead to a picture of anemia of chronic disease, with defective iron utilization, inappropriate erythropoietin responsiveness, and depressed bone marrow function.
The criteria for diagnosing absolute iron deficiency is a serum ferritin level <100 ng/mL, and the criteria for functional iron deficiency is a ferritin serum level between 100 and 300 ng/mL, combined with a transferrin saturation <20%.
Small studies have shown that erythropoiesis-stimulating agents (ESAs) improve subjective measures of HF. However, a large pivotal outcome trial found that the ESA darbepoetin alfa did not improve long-term outcomes in patients with HF with reduced ejection fraction, and instead was associated with adverse effects. Studies using IV iron showed better results, with improvements in subjective and objective end points.
32.1. Targeting Iron Deficiency and Anemia in Heart Failure
Oral iron supplementation is the standard therapy for patients with ID because it is convenient, readily available, and inexpensive.
However, in patients with HF, apart from gastrointestinal intolerance, oral iron is poorly absorbed because of elevated hepcidin, which inhibits iron absorption by reducing transmembrane ferroportin on enterocytes, thereby preventing iron transfer from enterocytes to the blood
[51][62].
With this background, it is unlikely that co-administration of vitamin C will increase gastrointestinal iron absorption.
Treating iron deficiency in HF with intravenous iron administration results in a reduction of HF-related hospitalizations and severe adverse events, and improves HF symptoms and quality of life
[42][43][53,54]. Treatment with intravenous ferric carboxymaltose in patients with chronic heart failure and iron deficiency, with or without anemia, improves symptoms, functional capacity, and quality of life
[52][63]. This prompted the ESC HF guidelines to recommend (II a) intravenous iron administration be considered in symptomatic HF with reduced ejection fraction patients
[53][64].
Gastrointestinal malabsorption, long-term aspirin use, and uremic gastritis may also precipitate iron-deficiency anemia. Though erythropoietin levels are elevated and correlate with disease severity in heart failure, the elevation is inadequate.
Small studies have suggested that increase in hemoglobin with an erythropoiesis-stimulating agent (ESA) may improve functional capacity and reduce hospitalization in patients with heart failure and anemia
[46][57]. Indeed, ESAs have not improved cardiovascular outcomes in anemic patients with chronic kidney disease, on the contrary it increased the risk of thrombotic events.
Swedberg et al. evaluated the effect of correcting anemia in patients with systolic heart failure with darbepoetin alfa
[54][65], which led to an increase in the hemoglobin level. Despite this improvement, the darbepoetin alfa did not reduce the risk of the primary outcome of death or hospitalization for worsening heart failure; however, more patients had fatal or nonfatal strokes in the darbepoetin alfa group than in the placebo group. Fatal or nonfatal stroke occurred in 3.7% in the study group, and 2.7% in the placebo group (
p = 0.23). In light of their findings, darbepoetin alfa is not recommended in this setting.
Similarly, the Trial to Reduce Cardiovascular Events with Aranesp Therapy (TREAT)
[55][66], showed a significant increase in thromboembolic events in patients receiving darbepoetin alfa.
32.2. Considerations after LVAD Implant and Potential Implications for Supplementation
Data on ID are lacking in patients who have transitioned toward LVAD. Despite the evident harmful effects of ID, and the positive results of intravenous iron administration in chronic HF patients, data in LVAD patients is limited. Gastrointestinal malabsorption due to right ventricular failure, long-term aspirin use, and uremic gastritis may also contribute to iron-deficiency anemia. Indeed, LVAD patients are at an increased risk of gastrointestinal blood loss: the vicious circle of iron deficiency can worsen the right ventricle function, increasing the risk of right ventricular failure (). Potentially, by early diagnosis and treating iron deficiency in LVAD patients, the right ventricle function might be optimized, preventing severe right ventricular failure, and improving patient outcomes. However, this should be further investigated.
Figure 3. Multiple causes of anemia in patients affected with heart failure and carrying LVAD, and potential impact of iron supplementation and erythropoietin.
Vrtovic et al. reviewed the data of 65 consecutive patients who underwent LVAD support for at least 6 months. Anemia, defined as hemoglobin levels <12 g/dL, was present in 30/65 patients (46%) after 6 months of LVAD support. Anemic patients had higher levels of pre-implant creatinine (1.8 + 0.8 vs. 1.4 + 0.5 mg/dL;
p = 0.04). The presence of anemia after 6 months correlated with higher levels of creatinine and blood urea nitrogen and lower levels of albumin. Multivariate Cox proportional hazards regression analysis revealed that levels of hemoglobin, creatinine, and albumin were associated with all-cause mortality at 15 months. Long-term survival was two times higher in non-anemic patients after 6 months of LVAD support than in anemic patients (
p = 0.01)
[56][67].
Use of ESAs in patients with LVADs may minimize blood transfusions and decrease allosensitization. The inflammatory milieu that ensues during LVAD support can suppress erythropoiesis and diminish its effectiveness. Anemic LVAD patients have lower-than-expected circulating erythropoietin levels. ESAs are interesting for patients listed for heart transplant, as reducing transfusions and subsequent risk of developing anti-HLA antibodies might delay procurement of an appropriate donor. Post-transplant, allosensitization can also lead to higher rates of organ rejection and allograft vasculopathy.
However, these potential benefits of ESAs in LVAD-supported patients should be compounded with their potential risks of thrombotic complications, which is concerning because LVADs are sensitive to pump thrombosis (PT). In a study by Nassif et al.
[57][68] ESA use in patients with the HeartMate II had higher rates of suspected PT (hazard ratio (HR): 2.35; 95% confidence interval (CI): 1.38 to 4.00;
p = 0.002). For every 100-unit increase in cumulative ESA dosage, the hazard of suspected PT increased by 10% (HR: 1.10; 95% CI: 1.04 to 1.16;
p < 0.001).
Mechanisms for development of pump thrombosis in patients receiving ESAs are likely multifactorial, and do not necessarily involve hyperviscosity.
On the basis of the currently available evidence, we envision a role for ESAs in the following scenarios:
- -
-
Preoperative anemic patients who are on IV anticoagulants, with the target of perioperative reduction of PRBC transfusions.
- -
-
Postoperative use started only when therapeutic anticoagulation is reached.
- -
-
During LVAD support in patients who require transfusions, and are still symptomatic, with renal failure and/or RVF.
Care should be taken according to the pump type: more liberal use might be justified with the HeartMate III in light of the lower rate of pump thrombosis.
The risk/benefit profile of ESA in anemic patients with GI bleeding and managed with low dose anticoagulation has yet to be determined.