Photoinduced Electron/Energy Transfer Reversible Addition-Fragmentation Chain-Transfer (PET-RAFT) polymerization, proposed for the first time in 2014, is based on an alternative activation of the thiocarbonylthio compounds through photoredox catalysts (PCs). This last presents significant advantages compared to other photochemical techniques in terms of applicability, cost, and sustainability.
- PET-RAFT
- controlled polymerization
- photochemistry
- advanced materials
- Photoinduced Reversible Addition-Fragmentation Chain Transfer Polymerization
1. Introduction
The introduction of reversible-deactivation radical polymerizations (RDRP) to the scientific community has provided relevant improvements over the classical radical polymerization technique thanks to the “living character” of this method. RDRP are based on the equilibrium between (macro) propagating radicals and dormant species through an activation/deactivation equilibrium [1]. The living chains spend most of their polymerization time in a dormant state and this allows to reach low dispersity (Đ), higher control over the molecular weight, and the possibility to continue the polymerization with fresh monomers in order to obtain complex architectures [2] (i.e., block, star, branched or hyper-branched network). RDRP is a collective term that embraces processes such as nitroxide-mediate polymerization (NMP) [3], atom transfer radical polymerization (ATRP) [4], and reversible addition-fragmentation chain-transfer polymerization (RAFT). In particular, RAFT follows a degenerative transfer mechanism in which there is no change in the overall number of radicals during the polymerization and it requires an external source of radicals, typically a radical initiator.
2. Form RAFT to PET-RAFT Polymerization
2.1. Reversible Addition-Fragmentation Chain-Transfer Polymerization (RAFT)
RAFT polymerization can be defined as one the most versatile of RDRP methods allowing the synthesis of complex architecture for a wide variety of functional monomers in different reaction conditions (aqueous or organic solution, bulk, dispersion, emulsion, miniemulsion) [5][6]. The overall process (
RAFT polymerization can be defined as one the most versatile of RDRP methods allowing the synthesis of complex architecture for a wide variety of functional monomers in different reaction conditions (aqueous or organic solution, bulk, dispersion, emulsion, miniemulsion) [5,6]. The overall process (
Figure 1) can be viewed as the insertion of monomer units between the S–R bond of the RAFT agent. The key features of the well-known RAFT polymerization mechanism [5][7][8] is the sequence of addition-fragmentation equilibrium shown in
) can be viewed as the insertion of monomer units between the S–R bond of the RAFT agent. The key features of the well-known RAFT polymerization mechanism [5,8,9] is the sequence of addition-fragmentation equilibrium shown in
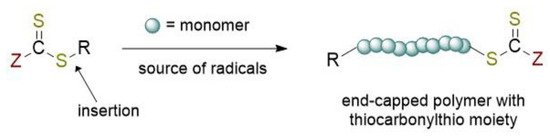
Figure 1.
Chemical structure of the RAFT agent and the overall insertion product of the reversible-addition chain transfer polymerization.
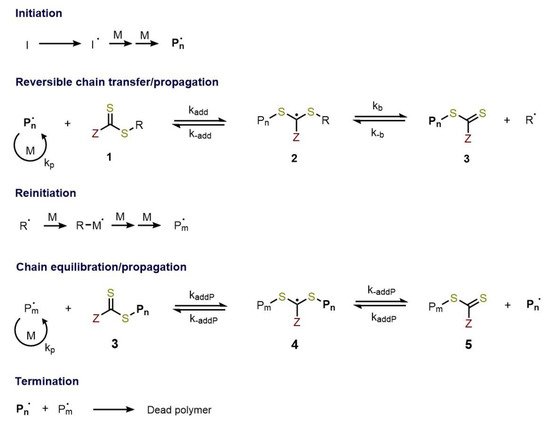
Figure 2.
Mechanism of RAFT polymerization.
2.2. Photoinduced Electron/Energy Transfer (PET)-RAFT Polymerization
Notwithstanding RAFT mechanism is well understood, the interaction between the excited photoredox catalyst (PC*) and the RAFT agent has not yet been well investigated. Two are nowadays the proposed mechanism [9][10] as shown in
Notwithstanding RAFT mechanism is well understood, the interaction between the excited photoredox catalyst (PC*) and the RAFT agent has not yet been well investigated. Two are nowadays the proposed mechanism [25,26] as shown in
.
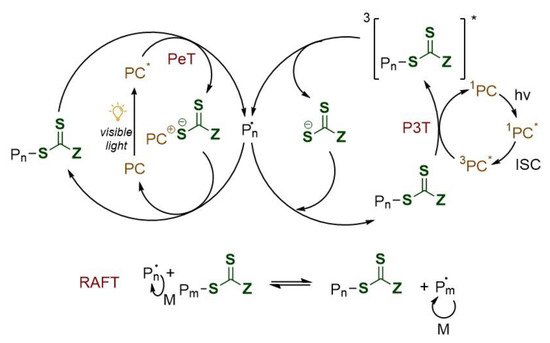
Figure 3.
Proposed mechanisms for PeT (
left
) and P3T (
right
) RAFT polymerization. PC: photocatalyst; PeT: photoinduced electron transfer; P3T: photoinduced triplet energy transfer (Dexter electron exchange); ISC: intersystem crossing; *: excited state.
The introduction of photoredox catalysts to initiate the polymerization has different advantages with respect to thermal initiated RAFT. Electromagnetic radiation guarantees spatiotemporal control and polymerization rate can be tuned by modulation of light intensity and wavelength. Furthermore, the reaction can be performed at room temperature and in presence of molecular oxygen. Finally, a very low amount of catalyst is needed (ppm range) in comparison with common thermal initiators concentration. In the following years, a wide variety of photocatalysts for PET-RAFT polymerization was studied (
) including naturally derived photoactive compounds or metal-free organic catalysts moving toward greener polymer manufacturing.
the main advantages and disadvantages of the most used catalysts are reported.
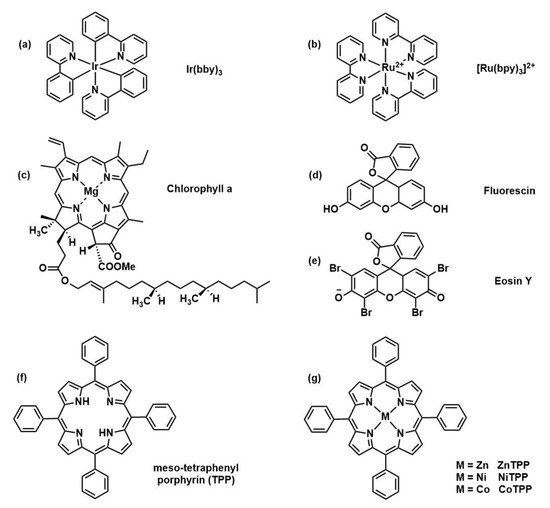
Figure 4.
Selection of catalyst structures: (Ir(ppy)
3) [11], (Ru(bpy)
) [23], (Ru(bpy)
3
Cl
2) [12], chlorophyll
) [30], chlorophyll
a [13], Fluorescine [14], Eosine Y [14], and (metallo)porphyrin-based structures [15].
[31], Fluorescine [32], Eosine Y [32], and (metallo)porphyrin-based structures [33].
Table 1.
| PC | Ref | Advantages | Disadvantages |
|---|
| (Ir(ppy) | 3) | [11] | [23] | Low energy absorption | Precious metal, expensive, soluble in few organic solvents | |||
| [Ru(bpy) | 3 | Cl | 2 | ] | [12] | [30] | Solubility in wide variety of solvents (also polar) | Precious metal, expensive |
| Porphyrin-based | [13][15][16] | [31,33,34] | Non precious metal, low-cost, low-toxicity, multiple absorption peaks | Presence of impurity, effective only with trithiocarbonates | ||||
| Eosine Y | [14] | [32] | Metal free, low cost | Sacrificial electron donor required, slow kinetics | ||||
| Metal oxides (ZnO, TiO | 2 | ) | [17][18] | [37,38] | Easy to recycle low-cost, low-toxicity | High energy irradiation | ||
| QDs | [19][20] | [41,42] | Easy to recycle, modulable absorption wavelength | Toxic compound | ||||
| Tertiary amines | [21] | [43] | Metal free | High energy irradiation |
3. PET-RAFT Polymerization: A Greener Approach
3.1. Light Sources
In principle, all the external light sources on the market are suitable for mediating photochemical reactions, including xenon, fluorescent lamps, mercury, halogen, LEDs, or even sunlight. Among them, light-emitting diodes (LEDs) have become dominant in photochemistry in the new millennium thanks to numerous advantages (
Figure 5). LEDs are based on electroluminescent emission, leading to a very precise emission wavelength with a narrow half width [22], which is pivotal for efficient polymerization. Very low voltage is required to power the lamp, which brings to less consumption of electricity, and less heat produced by the single compact LED units which may eliminate the need of heatsink or more complex equipment [23].
). LEDs are based on electroluminescent emission, leading to a very precise emission wavelength with a narrow half width [51], which is pivotal for efficient polymerization. Very low voltage is required to power the lamp, which brings to less consumption of electricity, and less heat produced by the single compact LED units which may eliminate the need of heatsink or more complex equipment [52].
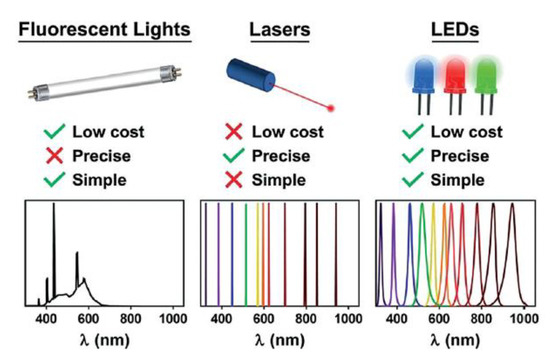
Figure 5. Main advantages of LED light sources. Reproduced with permission from ref. [24].
Main advantages of LED light sources. Reproduced with permission from ref. [50].
3.2. Recyclable Photocatalysts
over the past few years removal and recycle of the PC has become a hot topic for the scientific community (
Figure 7). For example, the use of catalysts anchored to solid support rather than in solution guarantees easy removal and recycle. Some relevant examples can be found in literature such as the use of celluloses as the supporting materials for the immobilization of ZnTPP [25][26] using simple grafting chemistry.
). For example, the use of catalysts anchored to solid support rather than in solution guarantees easy removal and recycle. Some relevant examples can be found in literature such as the use of celluloses as the supporting materials for the immobilization of ZnTPP [54,55] using simple grafting chemistry.
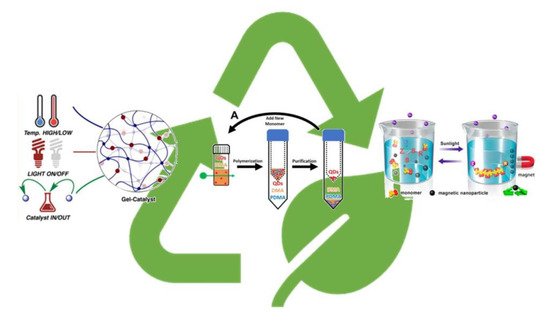
Figure 7. Recyclability of photoredox catalyst in PET-RAFT process. Adapted from refs. [27][28][29].
Recyclability of photoredox catalyst in PET-RAFT process. Adapted from refs. [57,58,59].
3.3. Oxygen Tolerance and High Throughput Polymerization
sually, in radical polymerization, to avoid termination reactions caused by the presence of oxygen, the reaction mixture is degassed (freeze-pump-thaw cycles) in sealed reactors and polymerization is conducted under an inert atmosphere (nitrogen or argon). Even if those methods are effective, they require time, inherently expensive gases, and complex equipment. PET-RAFT polymerization is a valuable alternative that can solve the problem in terms of applicability, cost, and sustainability.
3.4. Waste Minimization
An important concept of green chemistry relies on atom economy, meaning the ability of minimizing by-products, thus the reaction waste. Low by-products formation is possible using selective techniques, a feature that can be granted by the employment of photoredox catalysts. In fact, the electron transfer from the PC during irradiation is selectively accepted by the chain transfer agent, initiating the controlled polymerization. Moreover, light sources as LED [22], with narrow half width and low energy consumption, are safe and the visible radiation avoid the side reaction characteristic of high-energy UV or γ radiations such as the degradation of the CTA [30][31]. The direct generation of radical using the PC avoids the addition of exogenous radical sources which can give unwanted α or ω termini [32], dead chains polymers that remains as impurity in the synthesis of more complex architectures. Finally, the photocatalyst amount used on PET-RAFT remains in the ppm range and, as cited before, PCs recyclability is an hot topic in recent years. All these are interesting features which follow the green chemistry guidelines minimizing by-products formation.
An important concept of green chemistry relies on atom economy, meaning the ability of minimizing by-products, thus the reaction waste. Low by-products formation is possible using selective techniques, a feature that can be granted by the employment of photoredox catalysts. In fact, the electron transfer from the PC during irradiation is selectively accepted by the chain transfer agent, initiating the controlled polymerization. Moreover, light sources as LED [51], with narrow half width and low energy consumption, are safe and the visible radiation avoid the side reaction characteristic of high-energy UV or γ radiations such as the degradation of the CTA [19,20]. The direct generation of radical using the PC avoids the addition of exogenous radical sources which can give unwanted α or ω termini [24], dead chains polymers that remains as impurity in the synthesis of more complex architectures. Finally, the photocatalyst amount used on PET-RAFT remains in the ppm range and, as cited before, PCs recyclability is an hot topic in recent years. All these are interesting features which follow the green chemistry guidelines minimizing by-products formation.
4. Applications
Polymer science is nowadays pivotal for everyday life, thus covers a large portion of the actual market. One of the main challenges in polymer science is the development of new advanced materials with precise properties for multidisciplinary fields such as biotechnology, medical science, formulation chemistry, composite materials, microelectronics, plastic solar cells, biosensors, packaging, and so on. In this regard, photochemistry is a promising tool for developing innovative strategies appealing to both academic and industrial environments. The combination of photochemistry and RDRP provides good reaction control and does simplify elaborate reaction protocols. PET-RAFT polymerization is a valuable alternative and can solve the problem in terms of applicability, cost, and sustainability. Besides, the process is fully scalable thanks to the similarity with the traditional radical polymerization.
Polymer science is nowadays pivotal for everyday life, thus covers a large portion of the actual market. One of the main challenges in polymer science is the development of new advanced materials with precise properties for multidisciplinary fields such as biotechnology, medical science, formulation chemistry, composite materials, microelectronics, plastic solar cells, biosensors, packaging, and so on. In this regard, photochemistry is a promising tool for developing innovative strategies appealing to both academic and industrial environments. The combination of photochemistry and RDRP provides good reaction control and does simplify elaborate reaction protocols. PET-RAFT polymerization is a valuable alternative and can solve the problem in terms of applicability, cost, and sustainability. Besides, the process is fully scalable thanks to the similarity with the traditional radical polymerization.
