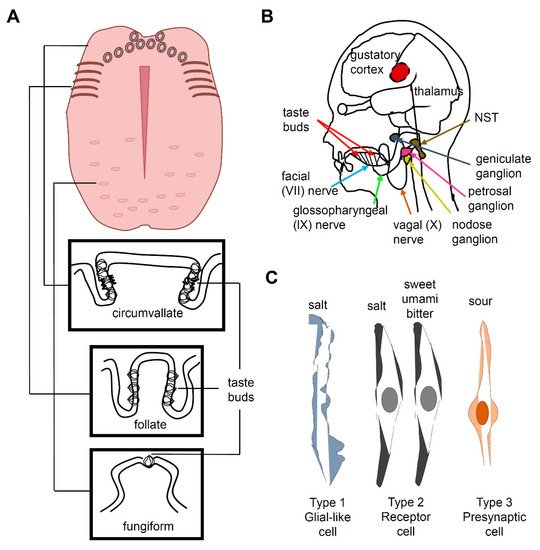
Taste buds, which are ovoid structures composed of 50–100 taste receptor cells (TRCs), are located in the tongue and palate epithelium. Circumvallate and foliate papillae contain dense groupings of taste buds at the back or sides of the tongue, respectively, whereas fungiform papillae contain scattered taste buds at the front of the tongue (A)
[4][7][4,7]. TRCs are neuroepithelial cells innervated by pseudounipolar neurons whose cell bodies are located at the petrosal and geniculate ganglia (B)
[7]. Three morphologically distinct types of cells (I, II, and III) are present in a taste bud and constitute five functional classes of sensory cells, each specialized to detect at least one of the five basic taste qualities, namely, sweet, umami, bitter, salty, and sour (C). Type I TRCs are glial-like cells and were earlier proposed to transduce an attractive salty taste
[8][9][8,9] Type II TRCs express T1R or T2R receptors. Sweet and umami taste receptors are heterodimers of T1R2 + T1R3 and T1R1 + T1R3, respectively
[10][11][12][10,11,12]. Bitter taste is mediated by T2Rs
[7][13][14][15][7,13,14,15]. Type III TRCs express otopetrin, a proton channel mediating sour taste
[16][17][18][16,17,18]. Type III TRCs also express PKD2L1 and carbonic anhydrase 4
[19][20][21][19,20,21]. However, the cell type associated with the perception of salty taste is controversial, because a recent study reported that sodium-taste cells can be considered as a Type II cell subset
[9]. The salty Type II TRCs are distinct from sweet-, umami-, and bitter-sensing Type II TRCs and are characterized by the absence of
Trpm5 and the presence of
ENaCα. The cells dedicated to attractive sodium taste are positive for ENaC and CALHM1/3
[8].
Most of the taste-related information is transmitted via the facial nerve (VII) and the glossopharyngeal nerve (IX) (B). The chorda tympani is a branch of the facial nerve that transmits taste messages from the anterior region of the tongue, which contains the fungiform papillae. The taste messages from the circumvallate papillae are transmitted via the glossopharyngeal nerve to the nucleus of the solitary tract (NST) in the medulla oblongata, while the foliate papillae send information via both VII and IX cranial nerves
[22][23][24][22,23,24]. When the superficial petrosal and glossopharyngeal nerves are abolished, the chorda tympani nerve occupies the space in the terminal field, which indicates that these two nerves compete or prune axon terminals to occupy the terminal space during development
[25]. The taste information in the NST passes to the thalamus, which projects to the cerebral cortex. The taste message is stored in the primary gustatory cortex in the insula and transported to the somatosensory cortex of the postcentral gyrus specifically dedicated to the tongue. This message is also carried to the prefrontal cortex, which coordinates taste association and perception of flavor.
Salt responses are recorded from the chorda tympani nerve
[18]. Depending on salt concentration, animals have two distinctive behaviors that are well reported in rodents: attraction (<100 mM) and aversion (>300 mM). At low concentrations, mice consume salt, but their behavior and neural responses are largely blocked by amiloride
[26]. In contrast, at high concentrations of salt, mice exhibit innate aversion, and these responses are unaffected by amiloride. Thus, a low concentration of salt is sensitive and a high concentration of salt is insensitive to the diuretic amiloride
[27]. Because epithelial Na
+ channels (ENaCs) are specifically inhibited by amiloride, they have been proposed to participate in low-concentration salt taste
[27][28][27,28]. ENaCs are non-voltage-gated and constitutively active channels that are highly selective of Na
+ and Li
+ over other monovalent cations such as K
+ [27]. An ENaC is composed of three subunits
[29], all of which are expressed in a small number of unique taste cells, with some being uncategorized TRCs
[3]. In humans, the salt taste is amiloride-insensitive
[30]. In addition to α, β, and γ ENaC subunits, humans express an additional δ ENaC subunit
[31]. Heterologous expression of fully functional human α ENaC and δ ENaC was achieved in
Xenopus oocytes
[31][32][33][31,32,33]. This may provide evidence that ENaC functions in salt sensation. Therefore, further studies are required to identify evolutionary changes in α, β, γ ENaCs in humans. ENaCs also play a major role in body fluid and blood pressure regulation
[34]. The δ-ENaC is also expressed in the brain, pancreas, testis, and ovary. Therefore, it is interesting to find any other roles of ENaC in different organs. Amiloride-insensitive high-salt transduction constitutes ~80% of the high-salt responses recorded in the chorda tympani nerve in mice
[35]. Furthermore, amiloride-insensitive high-salt responses are detected in type II TRCs, but not in type III TRCs in the fungiform papillae
[36]. In type II TRCs, the salt response is highly dependent on the presence of Cl
-, which is called “anion effect,” and involves the use of internal Ca
2+ stores, whereas type III TRCs utilize voltage-gated Ca
2+ channels activated by external Ca
2+. Cl
−, not Na
+, plays a major role in the amiloride-insensitive high-salt responses
[36], but the contribution of the cation remains unclear. In the circumvallate papilla, the amiloride-insensitive high salt response is mediated in type III TRCs through either an anion-selective or osmolality-sensitive channel
[30][37][30,37]. Recent studies in the field of salt sensation have shown substantial progress in our understanding of the effects of low and high salt concentrations on peripheral responses in the tongue. Therefore, future studies in this regard should be focused on determining how excessive salt intake and hypertension can be avoided using the salt taste sensation.